 Found 9 hits for monomerid = 50049254
Found 9 hits for monomerid = 50049254 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50049254
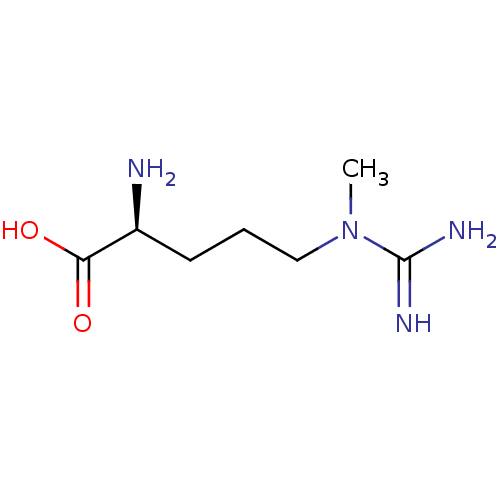
((2S)-2-amino-5-(N-methylcarbamimidamido)pentanoic ...)Show InChI InChI=1S/C7H16N4O2/c1-11(7(9)10)4-2-3-5(8)6(12)13/h5H,2-4,8H2,1H3,(H3,9,10)(H,12,13)/t5-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Binding affinity towards inducible nitric oxide synthase |
Bioorg Med Chem Lett 15: 3934-41 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.088
BindingDB Entry DOI: 10.7270/Q2V69KBC |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50049254

((2S)-2-amino-5-(N-methylcarbamimidamido)pentanoic ...)Show InChI InChI=1S/C7H16N4O2/c1-11(7(9)10)4-2-3-5(8)6(12)13/h5H,2-4,8H2,1H3,(H3,9,10)(H,12,13)/t5-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human inducible nitric oxide synthase |
J Med Chem 39: 669-72 (1996)
Article DOI: 10.1021/jm950766n
BindingDB Entry DOI: 10.7270/Q261110M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50049254

((2S)-2-amino-5-(N-methylcarbamimidamido)pentanoic ...)Show InChI InChI=1S/C7H16N4O2/c1-11(7(9)10)4-2-3-5(8)6(12)13/h5H,2-4,8H2,1H3,(H3,9,10)(H,12,13)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase |
J Med Chem 39: 669-72 (1996)
Article DOI: 10.1021/jm950766n
BindingDB Entry DOI: 10.7270/Q261110M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50049254

((2S)-2-amino-5-(N-methylcarbamimidamido)pentanoic ...)Show InChI InChI=1S/C7H16N4O2/c1-11(7(9)10)4-2-3-5(8)6(12)13/h5H,2-4,8H2,1H3,(H3,9,10)(H,12,13)/t5-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Endothelial nitric oxide synthase |
J Med Chem 39: 669-72 (1996)
Article DOI: 10.1021/jm950766n
BindingDB Entry DOI: 10.7270/Q261110M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50049254

((2S)-2-amino-5-(N-methylcarbamimidamido)pentanoic ...)Show InChI InChI=1S/C7H16N4O2/c1-11(7(9)10)4-2-3-5(8)6(12)13/h5H,2-4,8H2,1H3,(H3,9,10)(H,12,13)/t5-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Christian-Albrechts-University of Kiel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS by griess assay |
Bioorg Med Chem 16: 2305-12 (2008)
Article DOI: 10.1016/j.bmc.2007.11.066
BindingDB Entry DOI: 10.7270/Q2KW5GXN |
More data for this
Ligand-Target Pair | |
Dimethylarginine dimethylaminohydrolase 1 (DDAH)
(Homo sapiens (Human)) | BDBM50049254

((2S)-2-amino-5-(N-methylcarbamimidamido)pentanoic ...)Show InChI InChI=1S/C7H16N4O2/c1-11(7(9)10)4-2-3-5(8)6(12)13/h5H,2-4,8H2,1H3,(H3,9,10)(H,12,13)/t5-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Christian-Albrechts-University of Kiel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DDAH1 expressed in Escherichia coli BL21 cells |
Bioorg Med Chem 16: 2305-12 (2008)
Article DOI: 10.1016/j.bmc.2007.11.066
BindingDB Entry DOI: 10.7270/Q2KW5GXN |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50049254

((2S)-2-amino-5-(N-methylcarbamimidamido)pentanoic ...)Show InChI InChI=1S/C7H16N4O2/c1-11(7(9)10)4-2-3-5(8)6(12)13/h5H,2-4,8H2,1H3,(H3,9,10)(H,12,13)/t5-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Christian-Albrechts-University of Kiel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant eNOS by griess assay |
Bioorg Med Chem 16: 2305-12 (2008)
Article DOI: 10.1016/j.bmc.2007.11.066
BindingDB Entry DOI: 10.7270/Q2KW5GXN |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50049254

((2S)-2-amino-5-(N-methylcarbamimidamido)pentanoic ...)Show InChI InChI=1S/C7H16N4O2/c1-11(7(9)10)4-2-3-5(8)6(12)13/h5H,2-4,8H2,1H3,(H3,9,10)(H,12,13)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Christian-Albrechts-University of Kiel
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant nNOS by griess assay |
Bioorg Med Chem 16: 2305-12 (2008)
Article DOI: 10.1016/j.bmc.2007.11.066
BindingDB Entry DOI: 10.7270/Q2KW5GXN |
More data for this
Ligand-Target Pair | |
Nitric Oxide Synthase, inducible
(Mus musculus (mouse)) | BDBM50049254

((2S)-2-amino-5-(N-methylcarbamimidamido)pentanoic ...)Show InChI InChI=1S/C7H16N4O2/c1-11(7(9)10)4-2-3-5(8)6(12)13/h5H,2-4,8H2,1H3,(H3,9,10)(H,12,13)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Haute-Alsace
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against iNOS obtained from RAW 264.7 cells. |
Bioorg Med Chem Lett 8: 2961-6 (1999)
Article DOI: 10.1016/S0960-894X(98)00532-0
BindingDB Entry DOI: 10.7270/Q2DZ07G5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data