

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50049254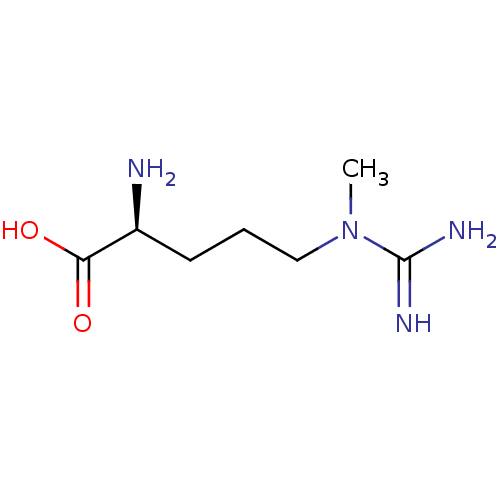 ((2S)-2-amino-5-(N-methylcarbamimidamido)pentanoic ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Binding affinity towards inducible nitric oxide synthase | Bioorg Med Chem Lett 15: 3934-41 (2005) Article DOI: 10.1016/j.bmcl.2005.05.088 BindingDB Entry DOI: 10.7270/Q2V69KBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50049254 ((2S)-2-amino-5-(N-methylcarbamimidamido)pentanoic ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human inducible nitric oxide synthase | J Med Chem 39: 669-72 (1996) Article DOI: 10.1021/jm950766n BindingDB Entry DOI: 10.7270/Q261110M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50049254 ((2S)-2-amino-5-(N-methylcarbamimidamido)pentanoic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Haute-Alsace Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against iNOS obtained from RAW 264.7 cells. | Bioorg Med Chem Lett 8: 2961-6 (1999) Article DOI: 10.1016/S0960-894X(98)00532-0 BindingDB Entry DOI: 10.7270/Q2DZ07G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50049254 ((2S)-2-amino-5-(N-methylcarbamimidamido)pentanoic ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Christian-Albrechts-University of Kiel Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS by griess assay | Bioorg Med Chem 16: 2305-12 (2008) Article DOI: 10.1016/j.bmc.2007.11.066 BindingDB Entry DOI: 10.7270/Q2KW5GXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||