

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50156282 CHEMBL3780847
SMILES: [H][C@@]12CC=C3C[C@@H](O)CC[C@]3(C)C1CC[C@@]1(C)C2CC=C1c1cccnc1
InChI Key: InChIKey=GZOSMCIZMLWJML-MOZXWMAFSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 17A1 (Homo sapiens (Human)) | BDBM50156282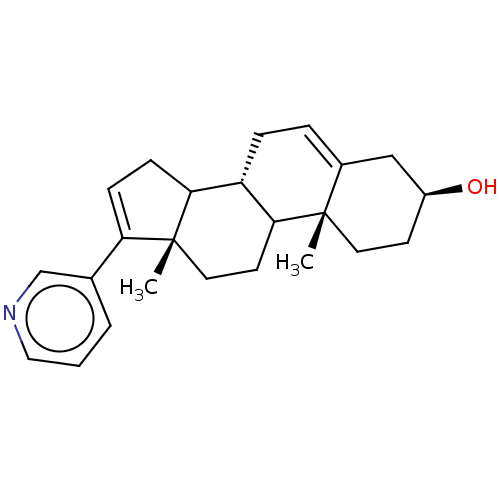 (CHEMBL3780847) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP17A1 in human adrenal microsomes using [3H]-pregnenolone as substrate incubated for 90 mins by scintillation proximity assay in pres... | ACS Med Chem Lett 7: 40-5 (2016) BindingDB Entry DOI: 10.7270/Q2TH8PK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Macaca fascicularis) | BDBM50156282 (CHEMBL3780847) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP17A1 in cynomolgus monkey adrenal microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity a... | ACS Med Chem Lett 7: 40-5 (2016) BindingDB Entry DOI: 10.7270/Q2TH8PK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50156282 (CHEMBL3780847) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 872 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP11B1 in human H295R cells using deoxycorticosterone as substrate incubated for 48 hrs by LC-MS method | ACS Med Chem Lett 7: 40-5 (2016) BindingDB Entry DOI: 10.7270/Q2TH8PK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 21 (Homo sapiens (Human)) | BDBM50156282 (CHEMBL3780847) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP21A2 in human H295R cells using hydroxy progesterone as substrate incubated for 120 mins by LC-MS method | ACS Med Chem Lett 7: 40-5 (2016) BindingDB Entry DOI: 10.7270/Q2TH8PK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Macaca fascicularis) | BDBM50156282 (CHEMBL3780847) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of CYP17A1 in cynomolgus monkey using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity assay in presence of... | ACS Med Chem Lett 7: 40-5 (2016) BindingDB Entry DOI: 10.7270/Q2TH8PK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||