

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50183627 CHEMBL206467::methyl(S)-1-(2-((S)-2-benzyl-2-hydroxy-3-((1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-ylamino)-3-oxopropyl)-2-(4-(pyridin-3-yl)benzyl)hydrazinyl)-3,3-dimethyl-1-oxobutan-2-ylcarbamate::{(1S)-1-[N'-[(2S)-2-HYDROXY-2-((1S,2R)-2-HYDROXY-INDAN-1-YLCARBAMOYL)-3-PHENYL-PROPYL]-N'-[4-(PYRIDINE-2-YL)-BENZYL]-HYDRAZINOCARBONYL]-2,2-DIMETHYL-PROPYL}-CARBAMIC ACID METHYL ESTER::{(1S)-1-[N'-[(2S)-2-hydroxy-2-((1S,2R)-2-hydroxy-indan-1-ylcarbamoyl)-3-phenyl-propyl]-N'-[4-(pyridine-3-yl)-benzyl]-hydrazinocarbonyl]-2,2-dimethyl-propyl}-carbamic acid methyl ester
SMILES: COC(=O)N[C@H](C(=O)NN(Cc1ccc(cc1)-c1cccnc1)C[C@@](O)(Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)C(C)(C)C
InChI Key: InChIKey=QPEXKJZILBODNL-NGXTUNLOSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50183627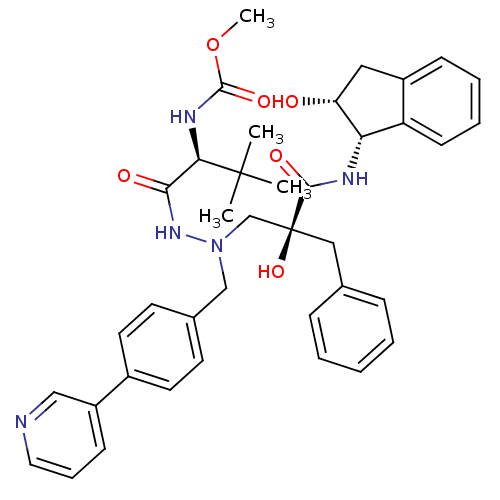 (CHEMBL206467 | methyl(S)-1-(2-((S)-2-benzyl-2-hydr...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM50183627 (CHEMBL206467 | methyl(S)-1-(2-((S)-2-benzyl-2-hydr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 49: 1828-32 (2006) Article DOI: 10.1021/jm051239z BindingDB Entry DOI: 10.7270/Q2PC3357 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||