

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50256329 1-(3-(3-mesitylureido)-2-naphthamido)cyclooctanecarboxylic acid::CHEMBL481534
SMILES: Cc1cc(C)c(NC(=O)Nc2cc3ccccc3cc2C(=O)NC2(CCCCCCC2)C(O)=O)c(C)c1
InChI Key: InChIKey=DRZBABLGPAMBFG-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycogen Phosphorylase (PYGL) (Homo sapiens (Human)) | BDBM50256329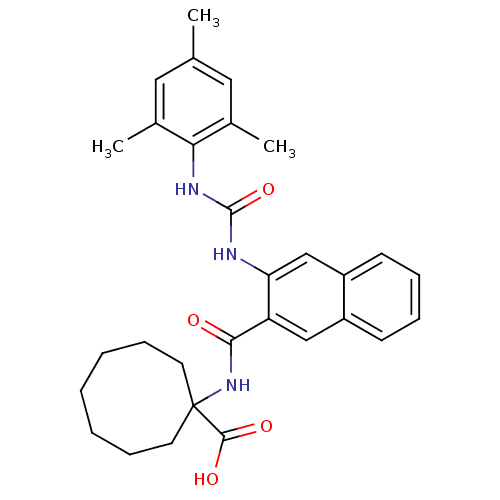 (1-(3-(3-mesitylureido)-2-naphthamido)cyclooctaneca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose | Bioorg Med Chem Lett 19: 976-80 (2009) Article DOI: 10.1016/j.bmcl.2008.11.085 BindingDB Entry DOI: 10.7270/Q26T0MGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50256329 (1-(3-(3-mesitylureido)-2-naphthamido)cyclooctaneca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | Bioorg Med Chem Lett 19: 976-80 (2009) Article DOI: 10.1016/j.bmcl.2008.11.085 BindingDB Entry DOI: 10.7270/Q26T0MGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen Phosphorylase (PYGL) (Homo sapiens (Human)) | BDBM50256329 (1-(3-(3-mesitylureido)-2-naphthamido)cyclooctaneca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 498 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human liver glycogen phosphorylase A in HepG2 cells assessed as forskolin-induced glycogenolysis | Bioorg Med Chem Lett 19: 976-80 (2009) Article DOI: 10.1016/j.bmcl.2008.11.085 BindingDB Entry DOI: 10.7270/Q26T0MGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||