

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50288363 CHEMBL4173216
SMILES: [H][C@@](NC(=O)c1cc2cccc(OC)c2o1)(C(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)NCc1c(C)cccc1C)[C@@]1([H])CCOC1
InChI Key: InChIKey=ZNVSERMHCCYPMY-JZILQDPNSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50288363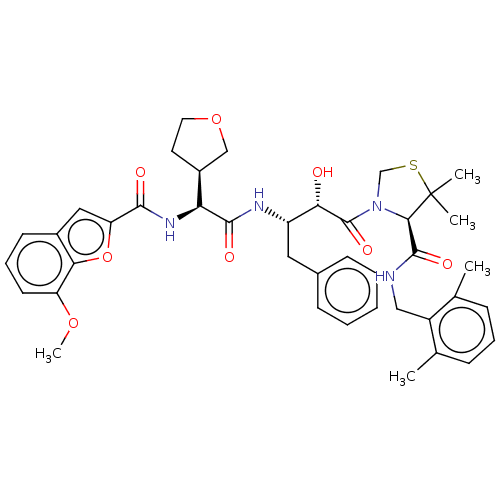 (CHEMBL4173216) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Kobe Gakuin University Curated by ChEMBL | Assay Description Inhibition of protease L10F/V32I/M46I/I47V/Q58E/I84V mutant in HIV1 A17 infected in human MT4 cells assessed as reduction in virus-induced cytopathic... | J Med Chem 61: 5138-5153 (2018) Article DOI: 10.1021/acs.jmedchem.7b01709 BindingDB Entry DOI: 10.7270/Q2T15651 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50288363 (CHEMBL4173216) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
Kobe Gakuin University Curated by ChEMBL | Assay Description Inhibition of DRV-resistant protease V32I/L33F/I54M/I84V mutant in HIV1 transfected in 293T cells after 48 hrs by lentiviral vector-based luciferase ... | J Med Chem 61: 5138-5153 (2018) Article DOI: 10.1021/acs.jmedchem.7b01709 BindingDB Entry DOI: 10.7270/Q2T15651 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50288363 (CHEMBL4173216) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Kobe Gakuin University Curated by ChEMBL | Assay Description Inhibition of DRV-resistant protease V32I/L33F/I54M/V82I mutant in HIV1 transfected in 293T cells after 48 hrs by lentiviral vector-based luciferase ... | J Med Chem 61: 5138-5153 (2018) Article DOI: 10.1021/acs.jmedchem.7b01709 BindingDB Entry DOI: 10.7270/Q2T15651 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||