

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: [NH-]C(=O)c1ccc[n+](CC(=O)N2CC[C@@H]3[C@H]2C(=O)N3S([O-])(=O)=O)c1
InChI Key: InChIKey=BYNLIVLZZWTJLF-KOLCDFICSA-M
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase (Escherichia coli) | BDBM50408523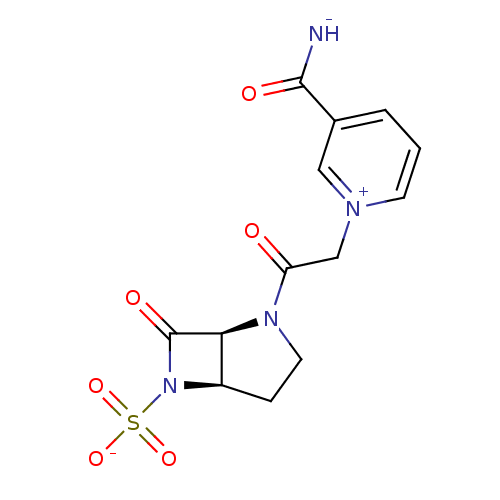 (CHEMBL2079714) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli TEM-3 Beta-lactamase | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50408523 (CHEMBL2079714) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | 0.00000900 | 0.900 | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Deacylation of 18SH Beta-lactamase of Pseudomonas aeruginosa | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50408523 (CHEMBL2079714) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition of P. aeruginosa 18SH Beta-lactamase. | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50408523 (CHEMBL2079714) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | 0.00000900 | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Time taken for deacylation of Pseudomonas aeruginosa 18SH Beta-lactamase | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50408523 (CHEMBL2079714) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 0.900 | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Acylation of Pseudomonas aeruginosa 18SH Beta-lactamase | J Med Chem 41: 3961-71 (1998) Article DOI: 10.1021/jm980023c BindingDB Entry DOI: 10.7270/Q2HT2NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||