

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50434157 CHEMBL2381958::US9340549, 78
SMILES: CC1(C)OC(=O)N([C@H]1c1ccccc1)c1ccc(cc1)C(=O)Nc1cccc2cccnc12
InChI Key: InChIKey=LUYWAGVRXKSNLL-DEOSSOPVSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tankyrase-1 (Homo sapiens (Human)) | BDBM50434157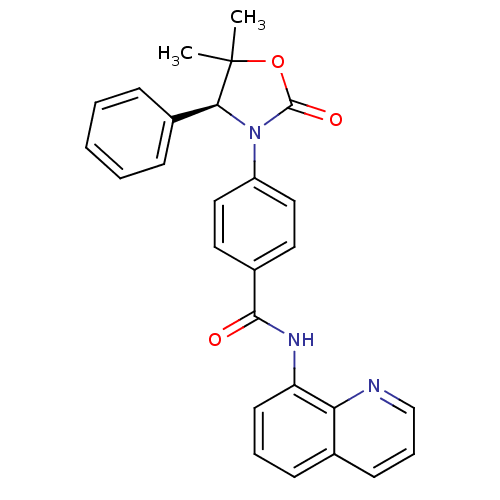 (CHEMBL2381958 | US9340549, 78) | PDB GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 198 | n/a | n/a | n/a | n/a | n/a | 25 |
AMGEN INC. US Patent | Assay Description The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T... | US Patent US9340549 (2016) BindingDB Entry DOI: 10.7270/Q2SN07VD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tankyrase-2 (Homo sapiens (Human)) | BDBM50434157 (CHEMBL2381958 | US9340549, 78) | PDB GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 189 | n/a | n/a | n/a | n/a | n/a | 25 |
AMGEN INC. US Patent | Assay Description The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T... | US Patent US9340549 (2016) BindingDB Entry DOI: 10.7270/Q2SN07VD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50434157 (CHEMBL2381958 | US9340549, 78) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of PARP1 (unknown origin) using histone as substrate after 1 hr by luminescence assay | J Med Chem 56: 4320-42 (2013) Article DOI: 10.1021/jm4000038 BindingDB Entry DOI: 10.7270/Q2QF8V74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human diphtheria toxin-like ADP-ribosyltransferase (ARTD3 or PARP3) (Homo sapiens (Human)) | BDBM50434157 (CHEMBL2381958 | US9340549, 78) | PDB MMDB KEGG B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of PARP3 (unknown origin) using histone as substrate after 1 hr by luminescence assay | J Med Chem 56: 4320-42 (2013) Article DOI: 10.1021/jm4000038 BindingDB Entry DOI: 10.7270/Q2QF8V74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50434157 (CHEMBL2381958 | US9340549, 78) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of PARP2 (unknown origin) using histone as substrate after 1 hr by luminescence assay | J Med Chem 56: 4320-42 (2013) Article DOI: 10.1021/jm4000038 BindingDB Entry DOI: 10.7270/Q2QF8V74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 6 (Homo sapiens (Human)) | BDBM50434157 (CHEMBL2381958 | US9340549, 78) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of PARP6 (unknown origin) using histone as substrate after 1 hr by luminescence assay | J Med Chem 56: 4320-42 (2013) Article DOI: 10.1021/jm4000038 BindingDB Entry DOI: 10.7270/Q2QF8V74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||