

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50504129 CHEMBL4521688
SMILES: [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@@H](C)CC)S(=O)(=O)c1ccc(cc1)[C@@H](O)CO
InChI Key: InChIKey=ZFBZVUBZCNSMDT-XXHYDFKDSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50504129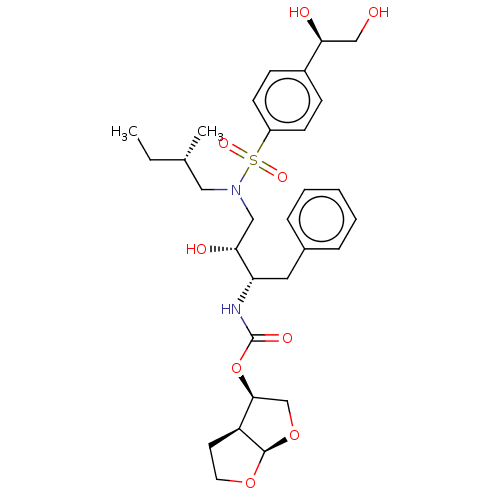 (CHEMBL4521688) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.216 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I84V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing natura... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||