 Found 12 hits for monomerid = 50526314
Found 12 hits for monomerid = 50526314 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Retinoic acid receptor RXR-alpha/Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM50526314
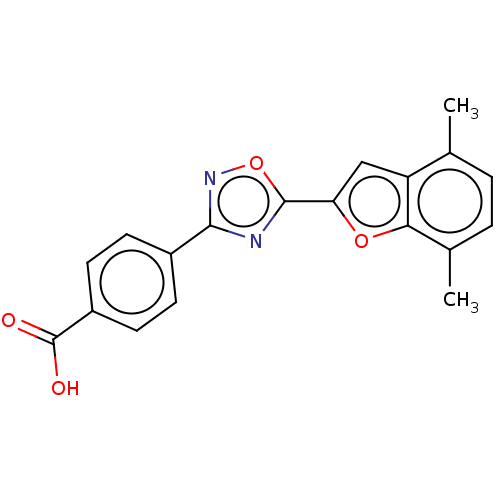
(CHEMBL4459692 | US10752616, Code No. BHBA-001)Show SMILES Cc1ccc(C)c2oc(cc12)-c1nc(no1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C19H14N2O4/c1-10-3-4-11(2)16-14(10)9-15(24-16)18-20-17(21-25-18)12-5-7-13(8-6-12)19(22)23/h3-9H,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 1.94 | n/a | n/a | n/a | n/a |
King''s College London
US Patent
| Assay Description
Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... |
US Patent US10752616 (2020)
|
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha/Retinoid X receptor alpha
(Homo sapiens (Human)) | BDBM50526314

(CHEMBL4459692 | US10752616, Code No. BHBA-001)Show SMILES Cc1ccc(C)c2oc(cc12)-c1nc(no1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C19H14N2O4/c1-10-3-4-11(2)16-14(10)9-15(24-16)18-20-17(21-25-18)12-5-7-13(8-6-12)19(22)23/h3-9H,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
King''s College London
US Patent
| Assay Description
Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... |
US Patent US10752616 (2020)
|
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/gamma
(Homo sapiens (Human)) | BDBM50526314

(CHEMBL4459692 | US10752616, Code No. BHBA-001)Show SMILES Cc1ccc(C)c2oc(cc12)-c1nc(no1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C19H14N2O4/c1-10-3-4-11(2)16-14(10)9-15(24-16)18-20-17(21-25-18)12-5-7-13(8-6-12)19(22)23/h3-9H,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
King''s College London
US Patent
| Assay Description
Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... |
US Patent US10752616 (2020)
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50526314

(CHEMBL4459692 | US10752616, Code No. BHBA-001)Show SMILES Cc1ccc(C)c2oc(cc12)-c1nc(no1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C19H14N2O4/c1-10-3-4-11(2)16-14(10)9-15(24-16)18-20-17(21-25-18)12-5-7-13(8-6-12)19(22)23/h3-9H,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes by LC-MS/MS analysis |
Bioorg Med Chem Lett 29: 995-1000 (2019)
Article DOI: 10.1016/j.bmcl.2019.02.011 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha/Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM50526314

(CHEMBL4459692 | US10752616, Code No. BHBA-001)Show SMILES Cc1ccc(C)c2oc(cc12)-c1nc(no1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C19H14N2O4/c1-10-3-4-11(2)16-14(10)9-15(24-16)18-20-17(21-25-18)12-5-7-13(8-6-12)19(22)23/h3-9H,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a |
King's College
Curated by ChEMBL
| Assay Description
Transactivation of GAL4 DBD-fused human RARbeta-LBD expressed in HEK293 cells by beta-lactamase reporter gene based assay |
Bioorg Med Chem Lett 29: 995-1000 (2019)
Article DOI: 10.1016/j.bmcl.2019.02.011 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50526314

(CHEMBL4459692 | US10752616, Code No. BHBA-001)Show SMILES Cc1ccc(C)c2oc(cc12)-c1nc(no1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C19H14N2O4/c1-10-3-4-11(2)16-14(10)9-15(24-16)18-20-17(21-25-18)12-5-7-13(8-6-12)19(22)23/h3-9H,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes by LC-MS/MS analysis |
Bioorg Med Chem Lett 29: 995-1000 (2019)
Article DOI: 10.1016/j.bmcl.2019.02.011 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Mus musculus) | BDBM50526314

(CHEMBL4459692 | US10752616, Code No. BHBA-001)Show SMILES Cc1ccc(C)c2oc(cc12)-c1nc(no1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C19H14N2O4/c1-10-3-4-11(2)16-14(10)9-15(24-16)18-20-17(21-25-18)12-5-7-13(8-6-12)19(22)23/h3-9H,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
King's College
Curated by ChEMBL
| Assay Description
Transactivation of GAL4-fused mouse RARgamma-LBD expressed in COS-7 cells after 24 hrs by bright-Glo reagent based assay |
Bioorg Med Chem Lett 29: 995-1000 (2019)
Article DOI: 10.1016/j.bmcl.2019.02.011 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A
(Homo sapiens (Human)) | BDBM50526314

(CHEMBL4459692 | US10752616, Code No. BHBA-001)Show SMILES Cc1ccc(C)c2oc(cc12)-c1nc(no1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C19H14N2O4/c1-10-3-4-11(2)16-14(10)9-15(24-16)18-20-17(21-25-18)12-5-7-13(8-6-12)19(22)23/h3-9H,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes by LC-MS/MS analysis |
Bioorg Med Chem Lett 29: 995-1000 (2019)
Article DOI: 10.1016/j.bmcl.2019.02.011 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50526314

(CHEMBL4459692 | US10752616, Code No. BHBA-001)Show SMILES Cc1ccc(C)c2oc(cc12)-c1nc(no1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C19H14N2O4/c1-10-3-4-11(2)16-14(10)9-15(24-16)18-20-17(21-25-18)12-5-7-13(8-6-12)19(22)23/h3-9H,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes by LC-MS/MS analysis |
Bioorg Med Chem Lett 29: 995-1000 (2019)
Article DOI: 10.1016/j.bmcl.2019.02.011 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50526314

(CHEMBL4459692 | US10752616, Code No. BHBA-001)Show SMILES Cc1ccc(C)c2oc(cc12)-c1nc(no1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C19H14N2O4/c1-10-3-4-11(2)16-14(10)9-15(24-16)18-20-17(21-25-18)12-5-7-13(8-6-12)19(22)23/h3-9H,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes by LC-MS/MS analysis |
Bioorg Med Chem Lett 29: 995-1000 (2019)
Article DOI: 10.1016/j.bmcl.2019.02.011 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Mus musculus) | BDBM50526314

(CHEMBL4459692 | US10752616, Code No. BHBA-001)Show SMILES Cc1ccc(C)c2oc(cc12)-c1nc(no1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C19H14N2O4/c1-10-3-4-11(2)16-14(10)9-15(24-16)18-20-17(21-25-18)12-5-7-13(8-6-12)19(22)23/h3-9H,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
King's College
Curated by ChEMBL
| Assay Description
Transactivation of GAL4-fused mouse RARbeta-LBD expressed in COS-7 cells after 24 hrs by bright-Glo reagent based assay |
Bioorg Med Chem Lett 29: 995-1000 (2019)
Article DOI: 10.1016/j.bmcl.2019.02.011 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Mus musculus) | BDBM50526314

(CHEMBL4459692 | US10752616, Code No. BHBA-001)Show SMILES Cc1ccc(C)c2oc(cc12)-c1nc(no1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C19H14N2O4/c1-10-3-4-11(2)16-14(10)9-15(24-16)18-20-17(21-25-18)12-5-7-13(8-6-12)19(22)23/h3-9H,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
King's College
Curated by ChEMBL
| Assay Description
Transactivation of GAL4-fused mouse RARalpha-LBD expressed in COS-7 cells after 24 hrs by bright-Glo reagent based assay |
Bioorg Med Chem Lett 29: 995-1000 (2019)
Article DOI: 10.1016/j.bmcl.2019.02.011 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data