

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50526727 CHEMBL4568508::US10807974, Example 100::US10995088, Example 100
SMILES: CS(=O)(=O)c1cc(cc(c1)S(=O)(=O)c1cnc(CN)s1)-c1ccccc1
InChI Key: InChIKey=HQPYHGXLGJSYMM-UHFFFAOYSA-N
Data: 13 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysyl oxidase homolog 2 (Homo sapiens (Human)) | BDBM50526727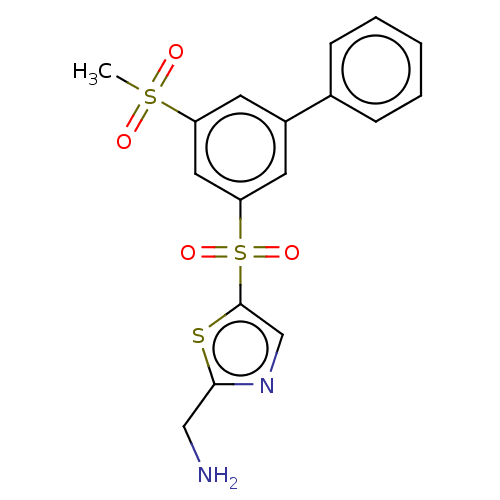 (CHEMBL4568508 | US10807974, Example 100 | US109950...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 587 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human C-terminal His10-tagged LOXL2 (Met1 to Gln774 residues) expressed in mouse NS0 cells using cadaverine hydrochloride as substrate ... | J Med Chem 63: 2308-2324 (2020) Article DOI: 10.1021/acs.jmedchem.9b01112 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysyl oxidase homolog 2 (Homo sapiens (Human)) | BDBM50526727 (CHEMBL4568508 | US10807974, Example 100 | US109950...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human C-terminal His10-tagged LOXL2 (Met1 to Gln774 residues) expressed in mouse NS0 cells using cadaverine hydrochloride as substrate ... | J Med Chem 63: 2308-2324 (2020) Article DOI: 10.1021/acs.jmedchem.9b01112 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50526727 (CHEMBL4568508 | US10807974, Example 100 | US109950...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOB expressed in baculovirus infected BTI insect cells using luminogenic MAO substrate measured after 1 hr by MAO-gl... | J Med Chem 63: 2308-2324 (2020) Article DOI: 10.1021/acs.jmedchem.9b01112 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase, copper containing (Homo sapiens (Human)) | BDBM50526727 (CHEMBL4568508 | US10807974, Example 100 | US109950...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of recombinant human SSAO expressed in CHO cells using luminogenic MAO substrate measured after 1 hr by MAO-glo assay | J Med Chem 63: 2308-2324 (2020) Article DOI: 10.1021/acs.jmedchem.9b01112 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM50526727 (CHEMBL4568508 | US10807974, Example 100 | US109950...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHOK1 cells by Qpatch electrophysiological assay | J Med Chem 63: 2308-2324 (2020) Article DOI: 10.1021/acs.jmedchem.9b01112 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-Amino Acid Oxidase (DAAO) (Sus scrofa (pig)) | BDBM50526727 (CHEMBL4568508 | US10807974, Example 100 | US109950...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of porcine kidney DAO measured after 1 hrs by ROS-glo assay | J Med Chem 63: 2308-2324 (2020) Article DOI: 10.1021/acs.jmedchem.9b01112 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50526727 (CHEMBL4568508 | US10807974, Example 100 | US109950...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INSTITUTE OF CANCER RESEARCH: ROYAL CANCER HOSPITAL US Patent | Assay Description ReagentsPromega ROS-Glo H2O2 Assay Kit, 50 ml. Cat No. G8821Inhibitors (Test Compounds):10 mM stock diluted to 10, 3, 1, 0.3, 0.1, 0.03, 0.01, 0.003,... | US Patent US10995088 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase (flavin-containing) A (Homo sapiens (Human)) | BDBM50526727 (CHEMBL4568508 | US10807974, Example 100 | US109950...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of recombinant human MAOA expressed in baculovirus infected BTI insect cells using luminogenic MAO substrate measured after 1 hr by MAO-gl... | J Med Chem 63: 2308-2324 (2020) Article DOI: 10.1021/acs.jmedchem.9b01112 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysyl oxidase homolog 2 (Homo sapiens (Human)) | BDBM50526727 (CHEMBL4568508 | US10807974, Example 100 | US109950...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human C-terminal His10-tagged LOXL2 (Met1 to Gln774 residues) expressed in mouse NS0 cells using cadaverine hydrochloride as substrate ... | J Med Chem 63: 2308-2324 (2020) Article DOI: 10.1021/acs.jmedchem.9b01112 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysyl oxidase homolog 2 (Homo sapiens (Human)) | BDBM50526727 (CHEMBL4568508 | US10807974, Example 100 | US109950...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research: Royal Cancer Hospital US Patent | Assay Description LOXL2 batch is obtained from R&D systems/Bio-techne. Incubate for 20 hours at room temp on a plate shaker. | US Patent US10807974 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diamine oxidase (Homo sapiens (Human)) | BDBM50526727 (CHEMBL4568508 | US10807974, Example 100 | US109950...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research: Royal Cancer Hospital US Patent | Assay Description Promega ROS-Glo H2O2 Assay Kit, 50 ml. Cat No. G8821. | US Patent US10807974 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysyl oxidase homolog 2 (Homo sapiens (Human)) | BDBM50526727 (CHEMBL4568508 | US10807974, Example 100 | US109950...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
THE INSTITUTE OF CANCER RESEARCH: ROYAL CANCER HOSPITAL US Patent | Assay Description 10. Incubate for 20 hrs at room temp on a plate shaker, protected from light. | US Patent US10995088 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysyl oxidase homolog 3 (Homo sapiens (Human)) | BDBM50526727 (CHEMBL4568508 | US10807974, Example 100 | US109950...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of recombinant C-terminal His-tagged human LOXL3 (1 to 753 residues) expressed in CHO cells using cadaverine hydrochloride as substrate pr... | J Med Chem 63: 2308-2324 (2020) Article DOI: 10.1021/acs.jmedchem.9b01112 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||