 Found 5 hits for monomerid = 50533571
Found 5 hits for monomerid = 50533571 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50533571
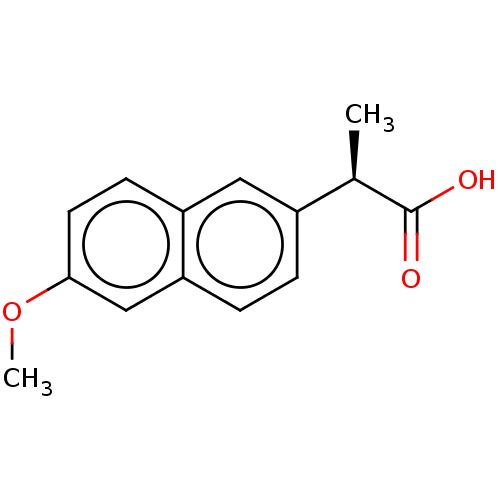
(CHEMBL1618254)Show InChI InChI=1S/C14H14O3/c1-9(14(15)16)10-3-4-12-8-13(17-2)6-5-11(12)7-10/h3-9H,1-2H3,(H,15,16)/t9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| PDB
PubMed
| 3.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR in presence of DHF and NADPH by UV-vis spectrometry by Lineweaver-Burk plot analysis |
J Med Chem 63: 8314-8324 (2020)
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase (cyclooxygenase)
(Ovis aries (Sheep)) | BDBM50533571

(CHEMBL1618254)Show InChI InChI=1S/C14H14O3/c1-9(14(15)16)10-3-4-12-8-13(17-2)6-5-11(12)7-10/h3-9H,1-2H3,(H,15,16)/t9-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in ram seminal vesicles using arachidonic acid as substrate assessed as reduction in PGH2 conversion to PGG2 by measuring TMPD oxi... |
J Med Chem 59: 7431-44 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00160 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50533571

(CHEMBL1618254)Show InChI InChI=1S/C14H14O3/c1-9(14(15)16)10-3-4-12-8-13(17-2)6-5-11(12)7-10/h3-9H,1-2H3,(H,15,16)/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AKR1C2 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... |
J Med Chem 59: 7431-44 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00160 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto-reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50533571

(CHEMBL1618254)Show InChI InChI=1S/C14H14O3/c1-9(14(15)16)10-3-4-12-8-13(17-2)6-5-11(12)7-10/h3-9H,1-2H3,(H,15,16)/t9-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AKR1C3 using S-tetralol as substrate assessed as reduction in NADP+-dependent S-tetralol oxidation preincubated for 1... |
J Med Chem 59: 7431-44 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00160 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E synthase/G/H synthase 2
(Homo sapiens (Human)) | BDBM50533571

(CHEMBL1618254)Show InChI InChI=1S/C14H14O3/c1-9(14(15)16)10-3-4-12-8-13(17-2)6-5-11(12)7-10/h3-9H,1-2H3,(H,15,16)/t9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) using arachidonic acid as substrate assessed as reduction in PGH2 conversion to PGG2 by measuring TMPD oxidation ... |
J Med Chem 59: 7431-44 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00160 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data