

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: [H][C@]12CN(C(C)=O)[C@]([H])(C[C@H]1c1ccc(cc1)-c1cc(cc3cc(ccc13)-c1ccc(cc1)C(F)(F)F)C(O)=O)C2
InChI Key:
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50584884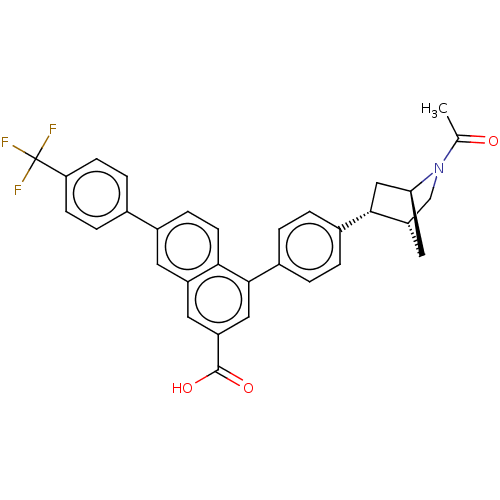 (CHEMBL5075741) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of adrenergic alpha 2B receptor (unknown origin) assessed as binding constant | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01964 BindingDB Entry DOI: 10.7270/Q2611470 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50584884 (CHEMBL5075741) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of dopamine D5 receptor (unknown origin) assessed as binding constant | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01964 BindingDB Entry DOI: 10.7270/Q2611470 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50584884 (CHEMBL5075741) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of sigma 2 receptor (unknown origin) assessed as binding constant | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01964 BindingDB Entry DOI: 10.7270/Q2611470 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50584884 (CHEMBL5075741) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of sigma 1 receptor (unknown origin) assessed as binding constant | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01964 BindingDB Entry DOI: 10.7270/Q2611470 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50584884 (CHEMBL5075741) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 64.1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of fluorescent antagonist binding to human P2Y14R expressed in CHO cells preincubated for 30 mins followed by 6-amino-9-(2-carboxy-4-((6-(... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01964 BindingDB Entry DOI: 10.7270/Q2611470 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50584884 (CHEMBL5075741) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of fluorescent antagonist binding to human P2Y14R expressed in CHO cells preincubated for 30 mins followed by 6-amino-9-(2-carboxy-4-((6-(... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01964 BindingDB Entry DOI: 10.7270/Q2611470 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||