

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Cc1[nH]c(\C=C2/C(=O)Nc3ncnc(Nc4ccc(F)c(Cl)c4)c23)c(C)c1CCC(O)=O
InChI Key: InChIKey=GDKHQUDZTDIVIZ-ZSOIEALJSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM5124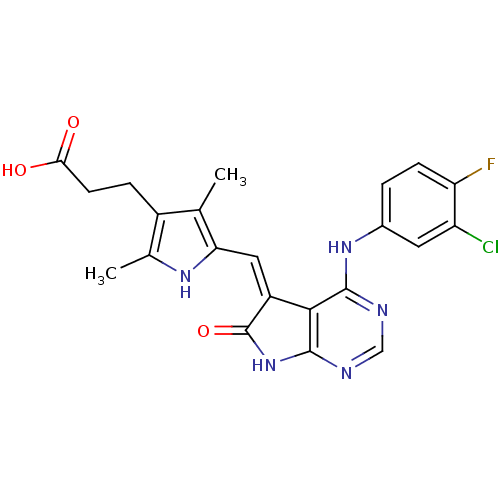 (3-(5-{[(5Z)-4-[(3-chloro-4-fluorophenyl)amino]-6-o...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
SUGEN, Inc. | Assay Description The assays were performed in 96-well microtiter plates that had been coated with a polyGluTyr peptide. Negative control wells received buffer alone w... | Bioorg Med Chem Lett 12: 2153-7 (2002) Article DOI: 10.1016/s0960-894x(02)00364-5 BindingDB Entry DOI: 10.7270/Q200009N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM5124 (3-(5-{[(5Z)-4-[(3-chloro-4-fluorophenyl)amino]-6-o...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SUGEN, Inc. | Assay Description IC50 is the inhibitor concentration which inhibits 50% of EGF-R or PDGFR-beta kinase autophosphorylation activity. The assay was performed in 96-well... | Bioorg Med Chem Lett 12: 2153-7 (2002) Article DOI: 10.1016/s0960-894x(02)00364-5 BindingDB Entry DOI: 10.7270/Q200009N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5124 (3-(5-{[(5Z)-4-[(3-chloro-4-fluorophenyl)amino]-6-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
SUGEN, Inc. | Assay Description IC50 is the inhibitor concentration which inhibits 50% of EGF-R or PDGFR-beta kinase autophosphorylation activity. The assay was performed in 96-well... | Bioorg Med Chem Lett 12: 2153-7 (2002) Article DOI: 10.1016/s0960-894x(02)00364-5 BindingDB Entry DOI: 10.7270/Q200009N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||