

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM8127 N-methyl-N-phenyl-4-pyrazolo[1,5-b]pyridazin-3-ylpyrimidin-2-amine::N-methyl-N-phenyl-4-{pyrazolo[1,5-a]pyridazin-3-yl}pyrimidin-2-amine::pyrazolo[1,5-b]pyridazine deriv. 17
SMILES: CN(c1ccccc1)c1nccc(n1)-c1cnn2ncccc12
InChI Key: InChIKey=XYEFJKRTJUALOK-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclin-Dependent Kinase 2 (CDK2) (Homo sapiens (Human)) | BDBM8127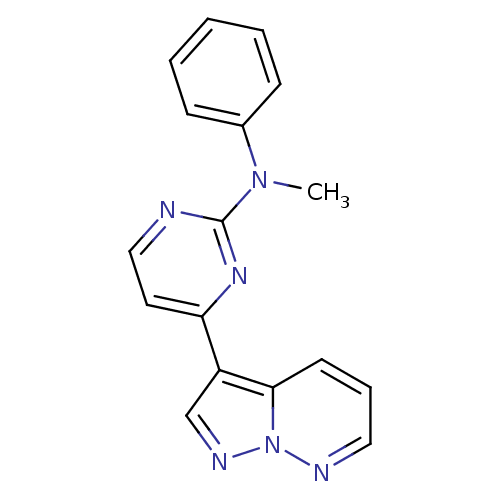 (N-methyl-N-phenyl-4-pyrazolo[1,5-b]pyridazin-3-ylp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.99E+4 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual-specificity tyrosine-phosphorylation regulated kinase 1A (Homo sapiens (Human)) | BDBM8127 (N-methyl-N-phenyl-4-pyrazolo[1,5-b]pyridazin-3-ylp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal Hex-tagged DYRK1A (127 to 485 residues) expressed in Escherichia coli BL21 (DE3) incubated for 1.5 hrs by ... | ACS Med Chem Lett 11: 1620-1626 (2020) | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8127 (N-methyl-N-phenyl-4-pyrazolo[1,5-b]pyridazin-3-ylp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||