

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM9046 Homodimeric Tacrine Analog 2c::N,N-Bis-(2,3-dihydro-1H-cyclopenta[1,2-b]quinolin-8-yl)-1,8-diaminooctane::N-(8-{1H,2H,3H-cyclopenta[b]quinolin-9-ylamino}octyl)-1H,2H,3H-cyclopenta[b]quinolin-9-amine
SMILES: C(CCCCNc1c2CCCc2nc2ccccc12)CCCNc1c2CCCc2nc2ccccc12
InChI Key: InChIKey=KBQNIGWBLLTLRQ-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9046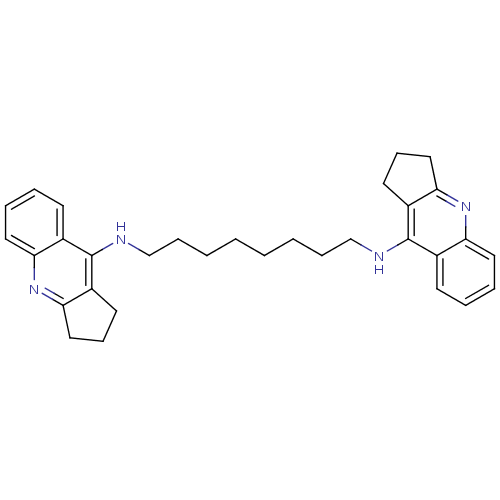 (Homodimeric Tacrine Analog 2c | N,N-Bis-(2,3-dihyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.4 | 37 |
National Defense Medical Center | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 45: 2277-82 (2002) Article DOI: 10.1021/jm010308g BindingDB Entry DOI: 10.7270/Q2KW5D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM9046 (Homodimeric Tacrine Analog 2c | N,N-Bis-(2,3-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University Curated by ChEMBL | Assay Description Inhibition of AChE | Eur J Med Chem 45: 1167-72 (2010) Article DOI: 10.1016/j.ejmech.2009.12.038 BindingDB Entry DOI: 10.7270/Q25H7GFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases (Homo sapiens (Human)) | BDBM9046 (Homodimeric Tacrine Analog 2c | N,N-Bis-(2,3-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
National Defense Medical Center | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 45: 2277-82 (2002) Article DOI: 10.1021/jm010308g BindingDB Entry DOI: 10.7270/Q2KW5D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||