

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Cc1ccc(\C=N\NC(=O)c2ccccc2Oc2ccccc2)o1
InChI Key: InChIKey=JJEDWBQZCRESJL-DEDYPNTBSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50436058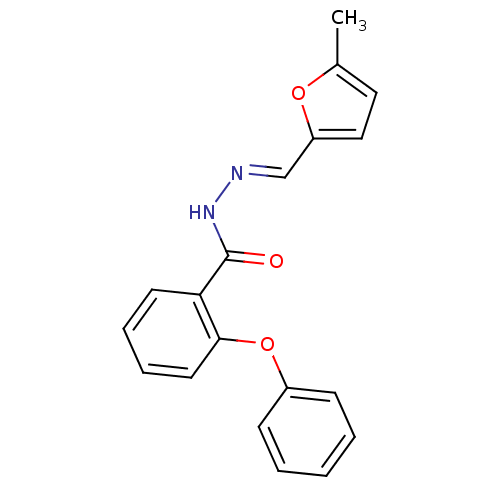 (CHEMBL254381 | PNU-74654 | US9738628, Compound PNU...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Inhibition of human wild-type beta-catenin (residues 138-686)/C-terminally fluorescein labeled human wild-type Tcf4 (residues 7-51) interaction after... | J Med Chem 58: 4678-92 (2015) Article DOI: 10.1021/acs.jmedchem.5b00223 BindingDB Entry DOI: 10.7270/Q2G44S28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50436058 (CHEMBL254381 | PNU-74654 | US9738628, Compound PNU...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a |
Chemical Genomics Centre of the Max Planck Society Curated by ChEMBL | Assay Description Inhibition of Tcf4 binding to beta-catenin (unknown origin) | Bioorg Med Chem 21: 4020-6 (2013) Article DOI: 10.1016/j.bmc.2013.02.050 BindingDB Entry DOI: 10.7270/Q2BP046Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 [138-686] (Homo sapiens (Human)) | BDBM50436058 (CHEMBL254381 | PNU-74654 | US9738628, Compound PNU...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | US Patent | 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Research Foundation US Patent | Assay Description Experiments were performed in 96-well Microfluor 2 black plates (Waltham, Mass.), and the samples were read by a Synergy 2 plate reader (Biotek, Wino... | US Patent US9738628 (2017) BindingDB Entry DOI: 10.7270/Q27P91H7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription factor 4 (Homo sapiens) | BDBM50436058 (CHEMBL254381 | PNU-74654 | US9738628, Compound PNU...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | n/a | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Affinity for CCK2 receptor assessed by inhibition of pentagastrin-stimulated acid secretion in perfused rat stomach | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription factor 4 (Homo sapiens) | BDBM50436058 (CHEMBL254381 | PNU-74654 | US9738628, Compound PNU...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a |
King Saud University Curated by ChEMBL | Assay Description Inhibition of His6 tagged Tcf4 1-53/beta catenin (unknown origin) interaction by VP-ITC titration calorimeter method | Bioorg Med Chem Lett 26: 1664-70 (2016) Article DOI: 10.1016/j.bmcl.2016.02.064 BindingDB Entry DOI: 10.7270/Q25H7K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50436058 (CHEMBL254381 | PNU-74654 | US9738628, Compound PNU...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00228 BindingDB Entry DOI: 10.7270/Q2GQ72Q3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 (Homo sapiens (Human)) | BDBM50436058 (CHEMBL254381 | PNU-74654 | US9738628, Compound PNU...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116920 BindingDB Entry DOI: 10.7270/Q2T157M8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Catenin beta-1 [138-686] (Homo sapiens (Human)) | BDBM50436058 (CHEMBL254381 | PNU-74654 | US9738628, Compound PNU...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | US Patent | 1.81E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Research Foundation US Patent | Assay Description Experiments were performed in white opaque 384-well plates from PerkinElmer (Waltham, Mass.), and the samples were read on a Synergy 2 plate reader (... | US Patent US9738628 (2017) BindingDB Entry DOI: 10.7270/Q27P91H7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||