

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50045698 1-Cyclopropyl-7-(2,6-dimethyl-pyridin-4-yl)-6,8-difluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid::CHEMBL8179::WIN-57294
SMILES: Cc1cc(cc(C)n1)-c1c(F)cc2c(c1F)n(cc(C(O)=O)c2=O)C1CC1
InChI Key: InChIKey=WHXJSJBKDGZVDA-UHFFFAOYSA-N
Data: 6 EC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA topoisomerase II (Homo sapiens (Human)) | BDBM50045698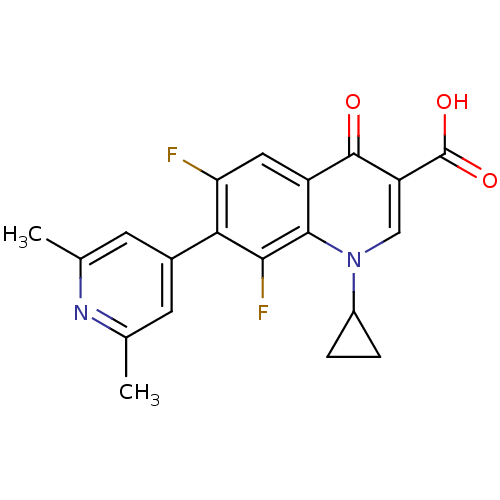 (1-Cyclopropyl-7-(2,6-dimethyl-pyridin-4-yl)-6,8-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | n/a | n/a | >5.40E+4 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of calf thymus DNA/ethidium bromide complex formation. | Bioorg Med Chem Lett 5: 405-410 (1995) Article DOI: 10.1016/0960-894X(95)00044-T BindingDB Entry DOI: 10.7270/Q2CZ37N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase II (Homo sapiens (Human)) | BDBM50045698 (1-Cyclopropyl-7-(2,6-dimethyl-pyridin-4-yl)-6,8-di...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | n/a | n/a | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against HeLa cell Topoisomerase II | Citation and Details BindingDB Entry DOI: 10.7270/Q2TX3HJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase II (Homo sapiens (Human)) | BDBM50045698 (1-Cyclopropyl-7-(2,6-dimethyl-pyridin-4-yl)-6,8-di...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity in a DNA cleavage assay using HeLa DNA topoisomerase II yielding its effective concentration | J Med Chem 45: 5564-75 (2002) Article DOI: 10.1021/jm010057b BindingDB Entry DOI: 10.7270/Q2XS5Z46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase II (Homo sapiens (Human)) | BDBM50045698 (1-Cyclopropyl-7-(2,6-dimethyl-pyridin-4-yl)-6,8-di...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for topoisomerase II inhibition in purified HeLa cells by SDS/K+ precipitation method | Bioorg Med Chem Lett 5: 405-410 (1995) Article DOI: 10.1016/0960-894X(95)00044-T BindingDB Entry DOI: 10.7270/Q2CZ37N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase II (Homo sapiens (Human)) | BDBM50045698 (1-Cyclopropyl-7-(2,6-dimethyl-pyridin-4-yl)-6,8-di...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Tested for inhibition of topoisomerase II isolated from HeLa cells by DNA-cleavage assay | J Med Chem 36: 2801-9 (1993) Article DOI: 10.1021/jm00071a010 BindingDB Entry DOI: 10.7270/Q2NK3H89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase II (Homo sapiens (Human)) | BDBM50045698 (1-Cyclopropyl-7-(2,6-dimethyl-pyridin-4-yl)-6,8-di...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | n/a | n/a | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity in a cell-free assay of DNA cleavage mediated by purified HeLa cell topoisomerase II. | Citation and Details BindingDB Entry DOI: 10.7270/Q2R49SXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||