 Found 1278 hits Enz. Inhib. hit(s) with Target = 'DNA topoisomerase 2'
Found 1278 hits Enz. Inhib. hit(s) with Target = 'DNA topoisomerase 2' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50366826
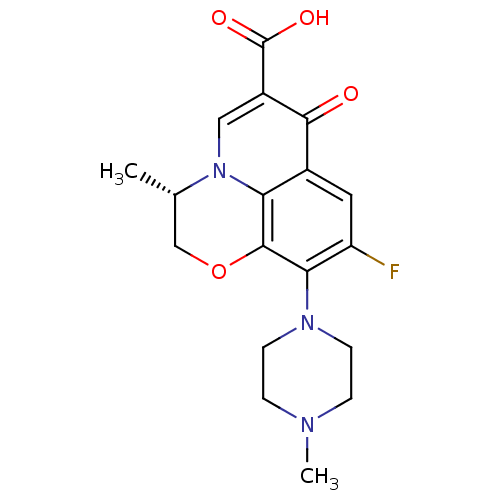
(DR-3355 | Floxacin | Iquix | LEVOFLOXACIN | Levaqu...)Show SMILES C[C@H]1COc2c(N3CCN(C)CC3)c(F)cc3c2n1cc(C(O)=O)c3=O |r| Show InChI InChI=1S/C18H20FN3O4/c1-10-9-26-17-14-11(16(23)12(18(24)25)8-22(10)14)7-13(19)15(17)21-5-3-20(2)4-6-21/h7-8,10H,3-6,9H2,1-2H3,(H,24,25)/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 2.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas University
Curated by ChEMBL
| Assay Description
Binding affinity against sigma receptor |
J Med Chem 30: 2283-6 (1987)
BindingDB Entry DOI: 10.7270/Q2R78HFX |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50045000
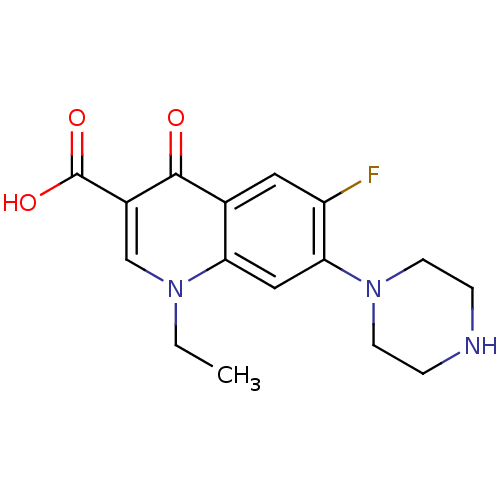
((NFLX)1-Ethyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,4-...)Show InChI InChI=1S/C16H18FN3O3/c1-2-19-9-11(16(22)23)15(21)10-7-12(17)14(8-13(10)19)20-5-3-18-4-6-20/h7-9,18H,2-6H2,1H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas University
Curated by ChEMBL
| Assay Description
Binding affinity against sigma receptor |
J Med Chem 30: 2283-6 (1987)
BindingDB Entry DOI: 10.7270/Q2R78HFX |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50045004
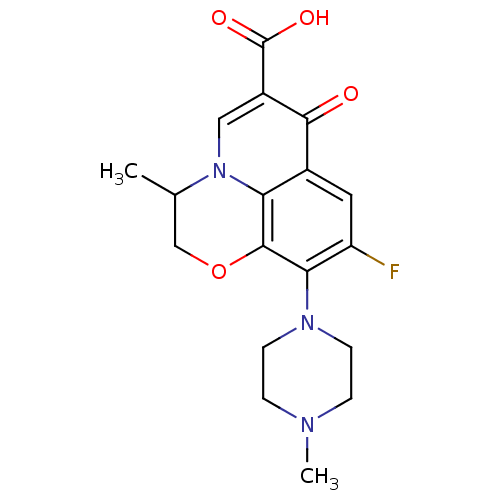
(9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-ox...)Show SMILES CC1COc2c(N3CCN(C)CC3)c(F)cc3c2n1cc(C(O)=O)c3=O Show InChI InChI=1S/C18H20FN3O4/c1-10-9-26-17-14-11(16(23)12(18(24)25)8-22(10)14)7-13(19)15(17)21-5-3-20(2)4-6-21/h7-8,10H,3-6,9H2,1-2H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 4.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas University
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone |
J Med Chem 30: 2283-6 (1987)
BindingDB Entry DOI: 10.7270/Q2R78HFX |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50142519
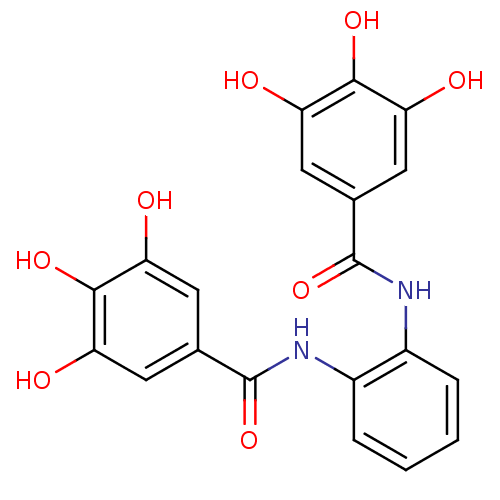
(CHEMBL47027 | N-[2-(3,4,5-triihydroxy-benzoylamino...)Show SMILES Oc1cc(cc(O)c1O)C(=O)Nc1ccccc1NC(=O)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C20H16N2O8/c23-13-5-9(6-14(24)17(13)27)19(29)21-11-3-1-2-4-12(11)22-20(30)10-7-15(25)18(28)16(26)8-10/h1-8,23-28H,(H,21,29)(H,22,30) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of relaxation activities of DNA topoisomerase II with respect to pBR322 DNA |
Bioorg Med Chem Lett 14: 1669-72 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.060
BindingDB Entry DOI: 10.7270/Q2GX4B0X |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50226409

(DEXTROFLOXACINE)Show SMILES C[C@@H]1COc2c(N3CCN(C)CC3)c(F)cc3c2n1cc(C(O)=O)c3=O |r| Show InChI InChI=1S/C18H20FN3O4/c1-10-9-26-17-14-11(16(23)12(18(24)25)8-22(10)14)7-13(19)15(17)21-5-3-20(2)4-6-21/h7-8,10H,3-6,9H2,1-2H3,(H,24,25)/t10-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 7.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas University
Curated by ChEMBL
| Assay Description
Binding affinity against sigma receptor |
J Med Chem 30: 2283-6 (1987)
BindingDB Entry DOI: 10.7270/Q2R78HFX |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50357677
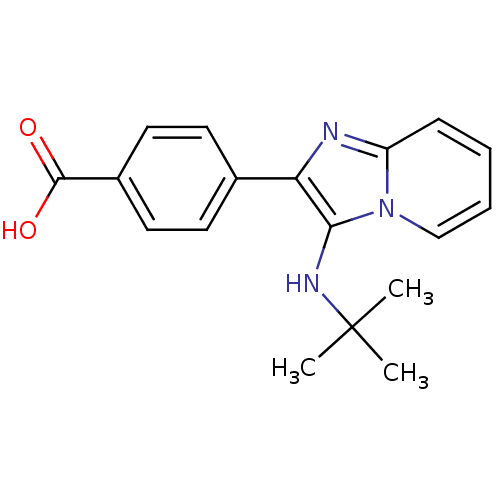
(CHEMBL1914951)Show InChI InChI=1S/C18H19N3O2/c1-18(2,3)20-16-15(19-14-6-4-5-11-21(14)16)12-7-9-13(10-8-12)17(22)23/h4-11,20H,1-3H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Competitive inhibition of human topoisomerase-2 alpha-ATPase activity by Lineweaver-Burk assay in presence of 200 to 520 uM ATP |
J Med Chem 54: 5013-30 (2011)
Article DOI: 10.1021/jm200235u
BindingDB Entry DOI: 10.7270/Q25T3KW8 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50366826

(DR-3355 | Floxacin | Iquix | LEVOFLOXACIN | Levaqu...)Show SMILES C[C@H]1COc2c(N3CCN(C)CC3)c(F)cc3c2n1cc(C(O)=O)c3=O |r| Show InChI InChI=1S/C18H20FN3O4/c1-10-9-26-17-14-11(16(23)12(18(24)25)8-22(10)14)7-13(19)15(17)21-5-3-20(2)4-6-21/h7-8,10H,3-6,9H2,1-2H3,(H,24,25)/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 9.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas University
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone |
J Med Chem 30: 2283-6 (1987)
BindingDB Entry DOI: 10.7270/Q2R78HFX |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50045000

((NFLX)1-Ethyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,4-...)Show InChI InChI=1S/C16H18FN3O3/c1-2-19-9-11(16(22)23)15(21)10-7-12(17)14(8-13(10)19)20-5-3-18-4-6-20/h7-9,18H,2-6H2,1H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas University
Curated by ChEMBL
| Assay Description
Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone |
J Med Chem 30: 2283-6 (1987)
BindingDB Entry DOI: 10.7270/Q2R78HFX |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50045004

(9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-ox...)Show SMILES CC1COc2c(N3CCN(C)CC3)c(F)cc3c2n1cc(C(O)=O)c3=O Show InChI InChI=1S/C18H20FN3O4/c1-10-9-26-17-14-11(16(23)12(18(24)25)8-22(10)14)7-13(19)15(17)21-5-3-20(2)4-6-21/h7-8,10H,3-6,9H2,1-2H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.32E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas University
Curated by ChEMBL
| Assay Description
Binding affinity against sigma receptor |
J Med Chem 30: 2283-6 (1987)
BindingDB Entry DOI: 10.7270/Q2R78HFX |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50226409

(DEXTROFLOXACINE)Show SMILES C[C@@H]1COc2c(N3CCN(C)CC3)c(F)cc3c2n1cc(C(O)=O)c3=O |r| Show InChI InChI=1S/C18H20FN3O4/c1-10-9-26-17-14-11(16(23)12(18(24)25)8-22(10)14)7-13(19)15(17)21-5-3-20(2)4-6-21/h7-8,10H,3-6,9H2,1-2H3,(H,24,25)/t10-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 2.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas University
Curated by ChEMBL
| Assay Description
Binding affinity against sigma receptor |
J Med Chem 30: 2283-6 (1987)
BindingDB Entry DOI: 10.7270/Q2R78HFX |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50449890

(CHEMBL4161876)Show SMILES COc1cc(cc(OC)c1OC)C1=N\C(=C/c2cc(Br)cc(Br)c2OC(C)=O)C(=O)NN1c1ncc(s1)-c1ccccc1 |t:13| Show InChI InChI=1S/C30H24Br2N4O6S/c1-16(37)42-26-18(10-20(31)14-21(26)32)11-22-29(38)35-36(30-33-15-25(43-30)17-8-6-5-7-9-17)28(34-22)19-12-23(39-2)27(41-4)24(13-19)40-3/h5-15H,1-4H3,(H,35,38)/b22-11- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Port Said University
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 2beta after 2 hrs by ELISA |
Eur J Med Chem 156: 563-579 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.003
BindingDB Entry DOI: 10.7270/Q20G3NPD |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50142519

(CHEMBL47027 | N-[2-(3,4,5-triihydroxy-benzoylamino...)Show SMILES Oc1cc(cc(O)c1O)C(=O)Nc1ccccc1NC(=O)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C20H16N2O8/c23-13-5-9(6-14(24)17(13)27)19(29)21-11-3-1-2-4-12(11)22-20(30)10-7-15(25)18(28)16(26)8-10/h1-8,23-28H,(H,21,29)(H,22,30) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against relaxation activity of DNA topoisomerase II by detecting the conversion of supercoiled pBR322 DNA to its relaxed for... |
Bioorg Med Chem Lett 14: 1669-72 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.060
BindingDB Entry DOI: 10.7270/Q2GX4B0X |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50449891

(CHEMBL4174756)Show SMILES COc1cc(cc(OC)c1OC)C1=N\C(=C/c2cc(Br)cc(Br)c2OC(C)=O)C(=O)NN1c1ccccc1 |t:13| Show InChI InChI=1S/C27H23Br2N3O6/c1-15(33)38-24-16(10-18(28)14-20(24)29)11-21-27(34)31-32(19-8-6-5-7-9-19)26(30-21)17-12-22(35-2)25(37-4)23(13-17)36-3/h5-14H,1-4H3,(H,31,34)/b21-11- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Port Said University
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 2beta after 2 hrs by ELISA |
Eur J Med Chem 156: 563-579 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.003
BindingDB Entry DOI: 10.7270/Q20G3NPD |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50470378

(CHEMBL108283)Show SMILES CNC(=O)OCc1cc(NC(=O)OC(C)(C)C)cc(Nc2c3ccccc3nc3ccccc23)c1 Show InChI InChI=1S/C27H28N4O4/c1-27(2,3)35-26(33)30-19-14-17(16-34-25(32)28-4)13-18(15-19)29-24-20-9-5-7-11-22(20)31-23-12-8-6-10-21(23)24/h5-15H,16H2,1-4H3,(H,28,32)(H,29,31)(H,30,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research
Curated by ChEMBL
| Assay Description
In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation |
J Med Chem 38: 3226-35 (1995)
Article DOI: 10.1021/jm00017a006
BindingDB Entry DOI: 10.7270/Q2XS5Z3R |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50410828

(CHEMBL5266387)Show SMILES [#7]-[#6](=O)-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)[C@@]([#7])([#6]-[#8])[#6]-[#16]-[#16]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C47H67N15O13S2/c48-35(65)15-14-29-39(69)59-32(20-36(49)66)42(72)60-33(44(74)62-17-5-9-34(62)43(73)57-28(8-4-16-54-46(51)52)38(68)55-21-37(50)67)22-76-77-24-47(53,23-63)45(75)61-31(19-26-10-12-27(64)13-11-26)41(71)58-30(40(70)56-29)18-25-6-2-1-3-7-25/h1-3,6-7,10-13,28-34,63-64H,4-5,8-9,14-24,53H2,(H2,48,65)(H2,49,66)(H2,50,67)(H,55,68)(H,56,70)(H,57,73)(H,58,71)(H,59,69)(H,60,72)(H,61,75)(H4,51,52,54)/t28-,29-,30-,31-,32-,33+,34-,47+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity in phenoxybenzamine-treated rat by Pressor assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50520631

(Ametantrone)Show SMILES OCCNCCNc1ccc(NCCNCCO)c2C(=O)c3ccccc3C(=O)c12 Show InChI InChI=1S/C22H28N4O4/c27-13-11-23-7-9-25-17-5-6-18(26-10-8-24-12-14-28)20-19(17)21(29)15-3-1-2-4-16(15)22(20)30/h1-6,23-28H,7-14H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Poison activity at recombinant human topoisomerase 2beta using pBR322 plasmid as substrate after 30 mins by ethidium bromide staining based agarose g... |
J Med Chem 61: 8947-8980 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01202
BindingDB Entry DOI: 10.7270/Q2H998M5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50449889

(CHEMBL4160802)Show SMILES COc1cc(cc(OC)c1OC)C1=N\C(=C/c2cc(Br)cc(Br)c2OC(C)=O)C(=O)NN1C(N)=S |t:13| Show InChI InChI=1S/C22H20Br2N4O6S/c1-10(29)34-18-11(5-13(23)9-14(18)24)6-15-21(30)27-28(22(25)35)20(26-15)12-7-16(31-2)19(33-4)17(8-12)32-3/h5-9H,1-4H3,(H2,25,35)(H,27,30)/b15-6- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Port Said University
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 2beta after 2 hrs by ELISA |
Eur J Med Chem 156: 563-579 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.003
BindingDB Entry DOI: 10.7270/Q20G3NPD |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50470365
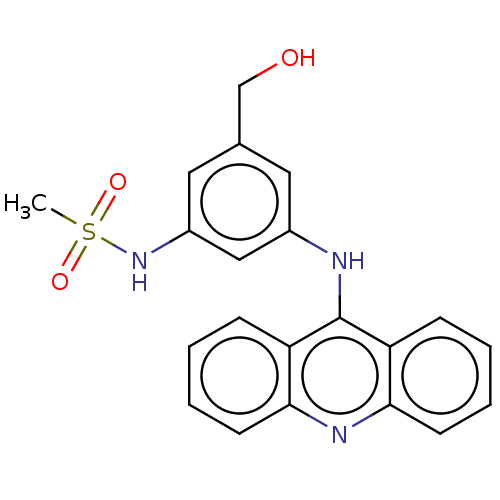
(CHEMBL326664)Show SMILES CS(=O)(=O)Nc1cc(CO)cc(Nc2c3ccccc3nc3ccccc23)c1 Show InChI InChI=1S/C21H19N3O3S/c1-28(26,27)24-16-11-14(13-25)10-15(12-16)22-21-17-6-2-4-8-19(17)23-20-9-5-3-7-18(20)21/h2-12,24-25H,13H2,1H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research
Curated by ChEMBL
| Assay Description
In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation |
J Med Chem 38: 3226-35 (1995)
Article DOI: 10.1021/jm00017a006
BindingDB Entry DOI: 10.7270/Q2XS5Z3R |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50470374

(CHEMBL322325)Show SMILES CN(C)CCCC(=O)Nc1cc(CO)cc(Nc2c3ccccc3nc3ccccc23)c1 Show InChI InChI=1S/C26H28N4O2/c1-30(2)13-7-12-25(32)27-19-14-18(17-31)15-20(16-19)28-26-21-8-3-5-10-23(21)29-24-11-6-4-9-22(24)26/h3-6,8-11,14-16,31H,7,12-13,17H2,1-2H3,(H,27,32)(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research
Curated by ChEMBL
| Assay Description
In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation |
J Med Chem 38: 3226-35 (1995)
Article DOI: 10.1021/jm00017a006
BindingDB Entry DOI: 10.7270/Q2XS5Z3R |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50123623
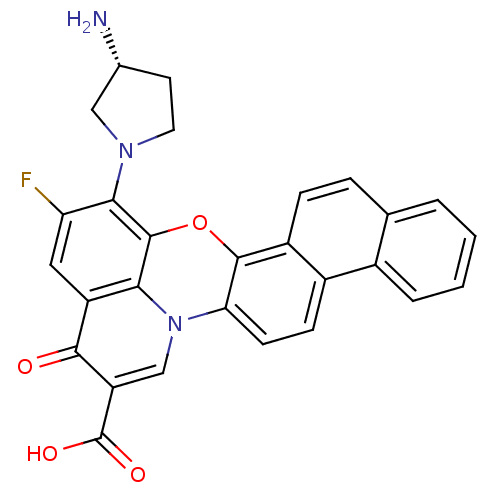
((R)-13-(3-aminopyrrolidin-1-yl)-12-fluoro-10-oxo-1...)Show SMILES N[C@@H]1CCN(C1)c1c(F)cc2c3c1oc1c4ccc5ccccc5c4ccc1n3cc(C(O)=O)c2=O Show InChI InChI=1S/C28H20FN3O4/c29-21-11-19-23-27(24(21)31-10-9-15(30)12-31)36-26-18-6-5-14-3-1-2-4-16(14)17(18)7-8-22(26)32(23)13-20(25(19)33)28(34)35/h1-8,11,13,15H,9-10,12,30H2,(H,34,35)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Toulouse
Curated by ChEMBL
| Assay Description
Induction of poison effect at DNA topoisomerase 2 by G-quadruplex interaction polymerase stop assay |
Bioorg Med Chem 17: 5396-407 (2009)
Article DOI: 10.1016/j.bmc.2009.06.053
BindingDB Entry DOI: 10.7270/Q21N816M |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50470371

(CHEMBL325543)Show SMILES CC(=O)OCc1cc(Nc2c3ccccc3nc3ccccc23)cc(NS(C)(=O)=O)c1 Show InChI InChI=1S/C23H21N3O4S/c1-15(27)30-14-16-11-17(13-18(12-16)26-31(2,28)29)24-23-19-7-3-5-9-21(19)25-22-10-6-4-8-20(22)23/h3-13,26H,14H2,1-2H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research
Curated by ChEMBL
| Assay Description
In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation |
J Med Chem 38: 3226-35 (1995)
Article DOI: 10.1021/jm00017a006
BindingDB Entry DOI: 10.7270/Q2XS5Z3R |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50127140

((-)-etoposide | (5S,5aR,8aR,9R)-9-(4-hydroxy-3,5-d...)Show SMILES COc1cc(cc(OC)c1O)[C@H]1[C@@H]2[C@H](COC2=O)[C@H](O[C@@H]2O[C@@H]3CO[C@@H](C)O[C@H]3[C@H](O)[C@H]2O)c2cc3OCOc3cc12 |r| Show InChI InChI=1S/C29H32O13/c1-11-36-9-20-27(40-11)24(31)25(32)29(41-20)42-26-14-7-17-16(38-10-39-17)6-13(14)21(22-15(26)8-37-28(22)33)12-4-18(34-2)23(30)19(5-12)35-3/h4-7,11,15,20-22,24-27,29-32H,8-10H2,1-3H3/t11-,15+,20-,21-,22+,24-,25-,26-,27-,29+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Port Said University
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 2beta after 2 hrs by ELISA |
Eur J Med Chem 156: 563-579 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.003
BindingDB Entry DOI: 10.7270/Q20G3NPD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50489101

(CHEMBL2296491)Show SMILES CCC(=O)NCCC1=C(SC)C(=O)c2nccc3c4ccccc4nc1c23 |c:7| Show InChI InChI=1S/C21H19N3O2S/c1-3-16(25)22-10-9-14-18-17-13(12-6-4-5-7-15(12)24-18)8-11-23-19(17)20(26)21(14)27-2/h4-8,11H,3,9-10H2,1-2H3,(H,22,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of DNA topoisomerase 2 (unknown origin) |
Citation and Details
Article DOI: 10.1007/s00044-013-0496-5
BindingDB Entry DOI: 10.7270/Q2VT1W17 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50449888

(CHEMBL4172196)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N\C(=C\c1cc(Br)cc(Br)c1OC(C)=O)C(=O)NNC(N)=S Show InChI InChI=1S/C22H22Br2N4O7S/c1-10(29)35-18-11(5-13(23)9-14(18)24)6-15(21(31)27-28-22(25)36)26-20(30)12-7-16(32-2)19(34-4)17(8-12)33-3/h5-9H,1-4H3,(H,26,30)(H,27,31)(H3,25,28,36)/b15-6+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Port Said University
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 2beta after 2 hrs by ELISA |
Eur J Med Chem 156: 563-579 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.003
BindingDB Entry DOI: 10.7270/Q20G3NPD |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50449887

(CHEMBL4169404)Show SMILES COc1cc(cc(OC)c1OC)C1=N\C(=C/c2cc(Br)cc(Br)c2OC(C)=O)C(=O)n2c(C)nc(=S)n12 |t:13| Show InChI InChI=1S/C24H20Br2N4O6S/c1-11-27-24(37)30-22(14-8-18(33-3)21(35-5)19(9-14)34-4)28-17(23(32)29(11)30)7-13-6-15(25)10-16(26)20(13)36-12(2)31/h6-10H,1-5H3/b17-7- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Port Said University
Curated by ChEMBL
| Assay Description
Inhibition of human DNA topoisomerase 2beta after 2 hrs by ELISA |
Eur J Med Chem 156: 563-579 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.003
BindingDB Entry DOI: 10.7270/Q20G3NPD |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50470361

(CHEMBL108404)Show SMILES CNC(=O)OCc1cc(N)cc(Nc2c3ccccc3nc3ccccc23)c1 Show InChI InChI=1S/C22H20N4O2/c1-24-22(27)28-13-14-10-15(23)12-16(11-14)25-21-17-6-2-4-8-19(17)26-20-9-5-3-7-18(20)21/h2-12H,13,23H2,1H3,(H,24,27)(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research
Curated by ChEMBL
| Assay Description
In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation |
J Med Chem 38: 3226-35 (1995)
Article DOI: 10.1021/jm00017a006
BindingDB Entry DOI: 10.7270/Q2XS5Z3R |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50489098
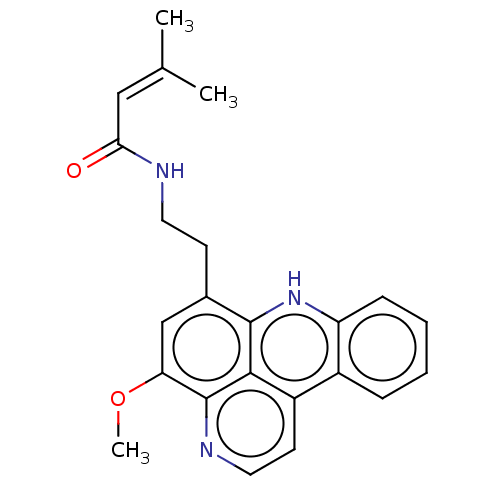
(CHEMBL2296492)Show SMILES COc1cc(CCNC(=O)C=C(C)C)c2[nH]c3ccccc3c3ccnc1c23 |(30.4,-27.22,;31.73,-26.45,;31.73,-24.91,;30.39,-24.14,;30.39,-22.6,;29.06,-21.82,;27.72,-22.58,;26.39,-21.8,;25.05,-22.57,;25.04,-24.11,;23.72,-21.79,;22.38,-22.55,;21.05,-21.77,;22.37,-24.09,;31.73,-21.84,;31.72,-20.32,;33.05,-19.53,;33.04,-18.01,;34.36,-17.24,;35.7,-18,;35.7,-19.53,;34.37,-20.3,;34.39,-21.83,;35.72,-22.59,;35.73,-24.14,;34.39,-24.91,;33.06,-24.14,;33.06,-22.6,)| Show InChI InChI=1S/C23H23N3O2/c1-14(2)12-20(27)24-10-8-15-13-19(28-3)23-21-17(9-11-25-23)16-6-4-5-7-18(16)26-22(15)21/h4-7,9,11-13,26H,8,10H2,1-3H3,(H,24,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of DNA topoisomerase 2 (unknown origin) |
Citation and Details
Article DOI: 10.1007/s00044-013-0496-5
BindingDB Entry DOI: 10.7270/Q2VT1W17 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50547613
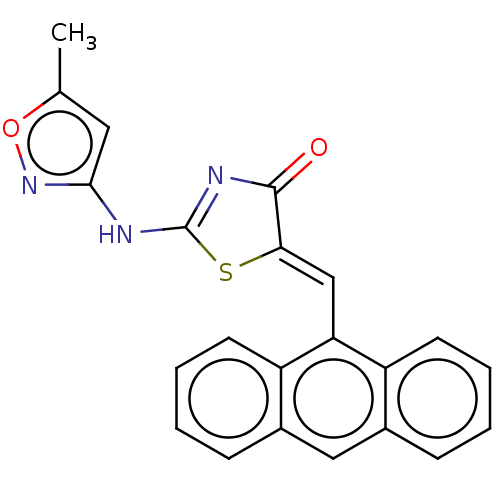
(CHEMBL4751362)Show SMILES Cc1cc(NC2=NC(=O)\C(S2)=C/c2c3ccccc3cc3ccccc23)no1 |t:5| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Topoisomerase II beta (unknown origin) relative to control |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115674
BindingDB Entry DOI: 10.7270/Q2TM7FQQ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50489096

(CHEMBL2296490)Show SMILES COC1=C(CCNC(C)=O)c2nc3ccccc3c3ccnc(C1=O)c23 |c:2| Show InChI InChI=1S/C20H17N3O3/c1-11(24)21-9-8-14-17-16-13(12-5-3-4-6-15(12)23-17)7-10-22-18(16)19(25)20(14)26-2/h3-7,10H,8-9H2,1-2H3,(H,21,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of DNA topoisomerase 2 (unknown origin) |
Citation and Details
Article DOI: 10.1007/s00044-013-0496-5
BindingDB Entry DOI: 10.7270/Q2VT1W17 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50142517
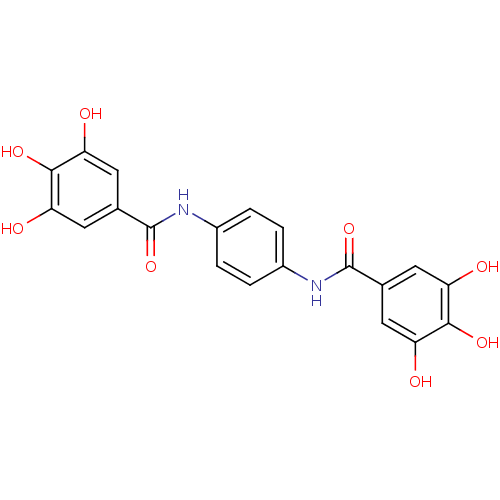
(CHEMBL47885 | N-[4-(3,4,5-triihydroxy-benzoylamino...)Show SMILES Oc1cc(cc(O)c1O)C(=O)Nc1ccc(NC(=O)c2cc(O)c(O)c(O)c2)cc1 Show InChI InChI=1S/C20H16N2O8/c23-13-5-9(6-14(24)17(13)27)19(29)21-11-1-2-12(4-3-11)22-20(30)10-7-15(25)18(28)16(26)8-10/h1-8,23-28H,(H,21,29)(H,22,30) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against relaxation activity of DNA topoisomerase II by detecting the conversion of supercoiled pBR322 DNA to its relaxed for... |
Bioorg Med Chem Lett 14: 1669-72 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.060
BindingDB Entry DOI: 10.7270/Q2GX4B0X |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50473498

(CHEMBL157831 | Ro-616653)Show SMILES COC(=O)c1c(Br)c(OC)cc(O)c1CSC[C@H](Nc1nc(cs1)-c1ccc(cc1)[N+]([O-])=O)c1nc(C)no1 |r| Show InChI InChI=1S/C24H22BrN5O7S2/c1-12-26-22(37-29-12)17(28-24-27-16(11-39-24)13-4-6-14(7-5-13)30(33)34)10-38-9-15-18(31)8-19(35-2)21(25)20(15)23(32)36-3/h4-8,11,17,31H,9-10H2,1-3H3,(H,27,28)/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 157 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antibacterial activity against Micrococcus luteus DNA gyrase. |
J Med Chem 44: 619-26 (2001)
Article DOI: 10.1021/jm0010623
BindingDB Entry DOI: 10.7270/Q2T43WT3 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50473498

(CHEMBL157831 | Ro-616653)Show SMILES COC(=O)c1c(Br)c(OC)cc(O)c1CSC[C@H](Nc1nc(cs1)-c1ccc(cc1)[N+]([O-])=O)c1nc(C)no1 |r| Show InChI InChI=1S/C24H22BrN5O7S2/c1-12-26-22(37-29-12)17(28-24-27-16(11-39-24)13-4-6-14(7-5-13)30(33)34)10-38-9-15-18(31)8-19(35-2)21(25)20(15)23(32)36-3/h4-8,11,17,31H,9-10H2,1-3H3,(H,27,28)/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 157 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antibacterial activity against Escherichia coli DNA gyrase |
J Med Chem 44: 619-26 (2001)
Article DOI: 10.1021/jm0010623
BindingDB Entry DOI: 10.7270/Q2T43WT3 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50505835
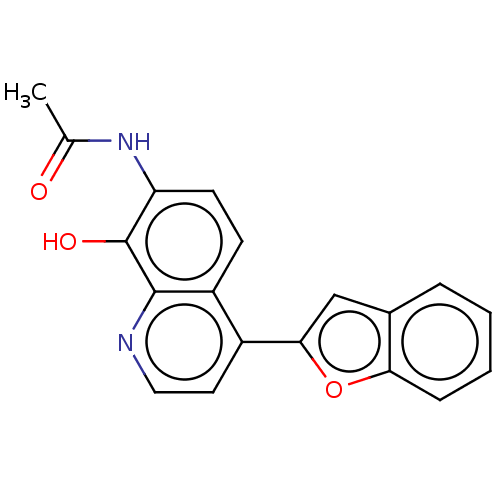
(CHEMBL4463643)Show InChI InChI=1S/C19H14N2O3/c1-11(22)21-15-7-6-14-13(8-9-20-18(14)19(15)23)17-10-12-4-2-3-5-16(12)24-17/h2-10,23H,1H3,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University
Curated by ChEMBL
| Assay Description
Inhibition of human topoisomerase 2alpha mediated decatenation using kDNA as susbtrate preincubated for 20 mins followed by substrate and ATP additio... |
J Med Chem 62: 10182-10203 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01065
BindingDB Entry DOI: 10.7270/Q2251NGG |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50142507

(CHEMBL47526 | N-[3-(3,4,5-triihydroxy-benzoylamino...)Show SMILES Oc1cc(cc(O)c1O)C(=O)Nc1cccc(NC(=O)c2cc(O)c(O)c(O)c2)c1 Show InChI InChI=1S/C20H16N2O8/c23-13-4-9(5-14(24)17(13)27)19(29)21-11-2-1-3-12(8-11)22-20(30)10-6-15(25)18(28)16(26)7-10/h1-8,23-28H,(H,21,29)(H,22,30) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against relaxation activity of DNA topoisomerase II by detecting the conversion of supercoiled pBR322 DNA to its relaxed for... |
Bioorg Med Chem Lett 14: 1669-72 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.060
BindingDB Entry DOI: 10.7270/Q2GX4B0X |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50470369

(CHEMBL325588)Show InChI InChI=1S/C20H16N2O/c23-13-14-6-5-7-15(12-14)21-20-16-8-1-3-10-18(16)22-19-11-4-2-9-17(19)20/h1-12,23H,13H2,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 226 | n/a | n/a | n/a | n/a | n/a | n/a |
Sloan-Kettering Institute for Cancer Research
Curated by ChEMBL
| Assay Description
In vitro 50% inhibition of topoisomerase II mediated k-DNA decatenation |
J Med Chem 38: 3226-35 (1995)
Article DOI: 10.1021/jm00017a006
BindingDB Entry DOI: 10.7270/Q2XS5Z3R |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50525581
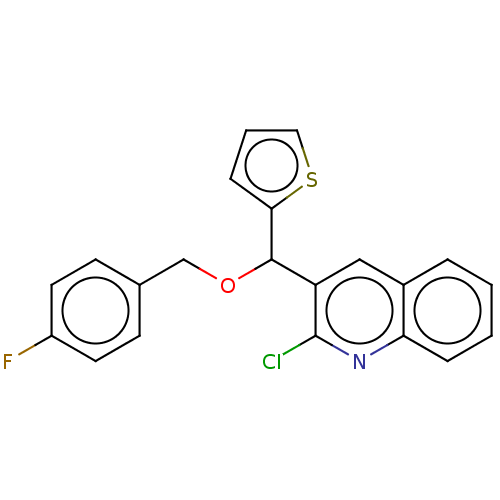
(CHEMBL4469453)Show InChI InChI=1S/C21H15ClFNOS/c22-21-17(12-15-4-1-2-5-18(15)24-21)20(19-6-3-11-26-19)25-13-14-7-9-16(23)10-8-14/h1-12,20H,13H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu Institute of Technology1-1 Sensuicho
Curated by ChEMBL
| Assay Description
Inhibition of DNA topoisomerase 2 in human MCF7 cells incubated for 18 to 24 hrs by kinase assay |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.07.042
BindingDB Entry DOI: 10.7270/Q2S46WDJ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50471898
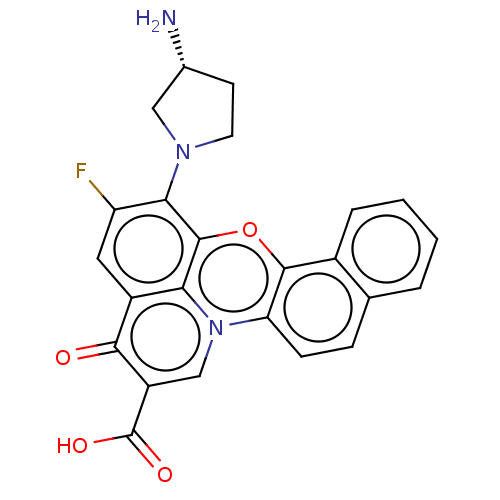
(CHEMBL135778)Show SMILES N[C@@H]1CCN(C1)c1c(F)cc2c3c1oc1c4ccccc4ccc1n3cc(C(O)=O)c2=O |r| Show InChI InChI=1S/C24H18FN3O4/c25-17-9-15-19-23(20(17)27-8-7-13(26)10-27)32-22-14-4-2-1-3-12(14)5-6-18(22)28(19)11-16(21(15)29)24(30)31/h1-6,9,11,13H,7-8,10,26H2,(H,30,31)/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase II as conversion of catenated to decatenated KDNA |
J Med Chem 41: 4273-8 (1998)
Article DOI: 10.1021/jm980265c
BindingDB Entry DOI: 10.7270/Q25X2CPR |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50473499

(BAY-507950 | CHEMBL156813)Show SMILES COC(=O)[C@H](CSCc1c(O)cc(OC)c(Br)c1C(=O)OC)Nc1nc(cs1)-c1ccc(cc1)[N+]([O-])=O |r| Show InChI InChI=1S/C23H22BrN3O8S2/c1-33-18-8-17(28)14(19(20(18)24)22(30)35-3)9-36-10-16(21(29)34-2)26-23-25-15(11-37-23)12-4-6-13(7-5-12)27(31)32/h4-8,11,16,28H,9-10H2,1-3H3,(H,25,26)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 326 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antibacterial activity against Micrococcus luteus DNA gyrase. |
J Med Chem 44: 619-26 (2001)
Article DOI: 10.1021/jm0010623
BindingDB Entry DOI: 10.7270/Q2T43WT3 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50487880
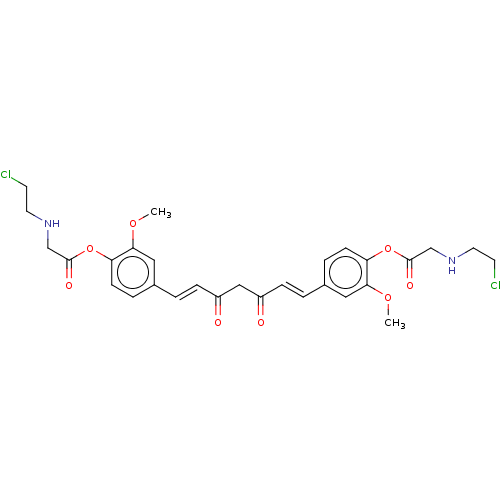
(CHEMBL2260090)Show SMILES COc1cc(\C=C\C(=O)CC(=O)\C=C\c2ccc(OC(=O)CNCCCl)c(OC)c2)ccc1OC(=O)CNCCCl Show InChI InChI=1S/C29H32Cl2N2O8/c1-38-26-15-20(5-9-24(26)40-28(36)18-32-13-11-30)3-7-22(34)17-23(35)8-4-21-6-10-25(27(16-21)39-2)41-29(37)19-33-14-12-31/h3-10,15-16,32-33H,11-14,17-19H2,1-2H3/b7-3+,8-4+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Homo sapiens (human) topoisomerase 2 assessed as decatenation of KDNA by agarose gel electrophoresis |
Citation and Details
Article DOI: 10.1007/s00044-011-9587-3
BindingDB Entry DOI: 10.7270/Q21Z47BG |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM15236

(3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...)Show SMILES Oc1cc(O)c2c(c1)oc(-c1cc(O)c(O)c(O)c1)c(O)c2=O Show InChI InChI=1S/C15H10O8/c16-6-3-7(17)11-10(4-6)23-15(14(22)13(11)21)5-1-8(18)12(20)9(19)2-5/h1-4,16-20,22H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Homo sapiens (human) DNA topoisomerase 2 |
Citation and Details
Article DOI: 10.1007/s00044-009-9233-5
BindingDB Entry DOI: 10.7270/Q27M0BVB |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50471897

(CHEMBL137098)Show SMILES N[C@@H]1CCN(C1)c1c(F)cc2c3c1oc1cc4ccccc4cc1n3cc(C(O)=O)c2=O Show InChI InChI=1S/C24H18FN3O4/c25-17-9-15-20-23(21(17)27-6-5-14(26)10-27)32-19-8-13-4-2-1-3-12(13)7-18(19)28(20)11-16(22(15)29)24(30)31/h1-4,7-9,11,14H,5-6,10,26H2,(H,30,31)/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase II as conversion of catenated to decatenated KDNA |
J Med Chem 41: 4273-8 (1998)
Article DOI: 10.1021/jm980265c
BindingDB Entry DOI: 10.7270/Q25X2CPR |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
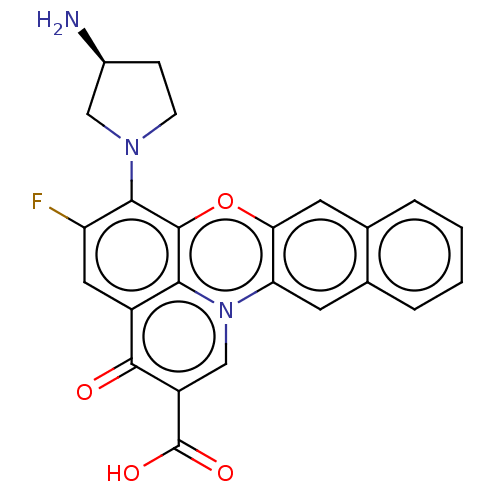
(Homo sapiens (Human)) | BDBM50471907
(CHEMBL337526)Show SMILES N[C@H]1CCN(C1)c1c(F)cc2c3c1oc1cc4ccccc4cc1n3cc(C(O)=O)c2=O Show InChI InChI=1S/C24H18FN3O4/c25-17-9-15-20-23(21(17)27-6-5-14(26)10-27)32-19-8-13-4-2-1-3-12(13)7-18(19)28(20)11-16(22(15)29)24(30)31/h1-4,7-9,11,14H,5-6,10,26H2,(H,30,31)/t14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase II as conversion of catenated to decatenated KDNA |
J Med Chem 41: 4273-8 (1998)
Article DOI: 10.1021/jm980265c
BindingDB Entry DOI: 10.7270/Q25X2CPR |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50525582
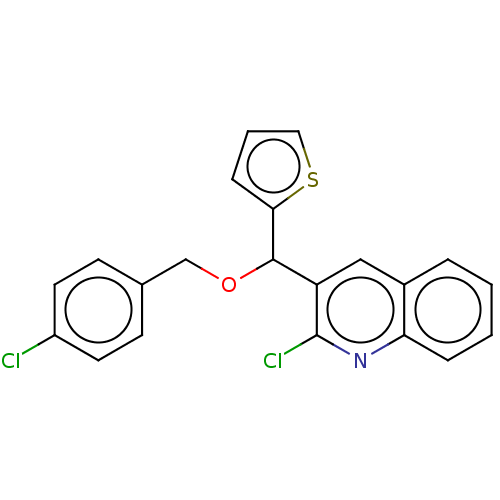
(CHEMBL4470431)Show InChI InChI=1S/C21H15Cl2NOS/c22-16-9-7-14(8-10-16)13-25-20(19-6-3-11-26-19)17-12-15-4-1-2-5-18(15)24-21(17)23/h1-12,20H,13H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu Institute of Technology1-1 Sensuicho
Curated by ChEMBL
| Assay Description
Inhibition of DNA topoisomerase 2 in human MCF7 cells incubated for 18 to 24 hrs by kinase assay |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.07.042
BindingDB Entry DOI: 10.7270/Q2S46WDJ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50229974

(CHEMBL178077)Show SMILES OC(=O)c1c2scc3COc4c(N5CCNCC5)c(F)cc(c4n23)c1=O Show InChI InChI=1S/C17H14FN3O4S/c18-10-5-9-12-15(13(10)20-3-1-19-2-4-20)25-6-8-7-26-16(21(8)12)11(14(9)22)17(23)24/h5,7,19H,1-4,6H2,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 452 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
The inhibitory activity was measured for the supercoiling activity of DNA gyrase isolated from Escherichia coli K-12 C600. |
J Med Chem 35: 94-9 (1992)
BindingDB Entry DOI: 10.7270/Q2D79DNN |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50008935
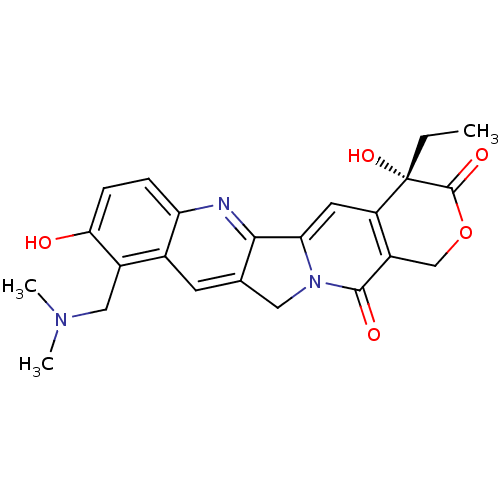
((20S)-10-Dimethylaminomethyl-4-ethyl-4,9-dihydroxy...)Show SMILES CC[C@@]1(O)C(=O)OCc2c1cc1-c3nc4ccc(O)c(CN(C)C)c4cc3Cn1c2=O |r| Show InChI InChI=1S/C23H23N3O5/c1-4-23(30)16-8-18-20-12(9-26(18)21(28)15(16)11-31-22(23)29)7-13-14(10-25(2)3)19(27)6-5-17(13)24-20/h5-8,27,30H,4,9-11H2,1-3H3/t23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu Institute of Technology1-1 Sensuicho
Curated by ChEMBL
| Assay Description
Inhibition of DNA topoisomerase 2 in human MCF7 cells incubated for 18 to 24 hrs by kinase assay |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.07.042
BindingDB Entry DOI: 10.7270/Q2S46WDJ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50473499

(BAY-507950 | CHEMBL156813)Show SMILES COC(=O)[C@H](CSCc1c(O)cc(OC)c(Br)c1C(=O)OC)Nc1nc(cs1)-c1ccc(cc1)[N+]([O-])=O |r| Show InChI InChI=1S/C23H22BrN3O8S2/c1-33-18-8-17(28)14(19(20(18)24)22(30)35-3)9-36-10-16(21(29)34-2)26-23-25-15(11-37-23)12-4-6-13(7-5-12)27(31)32/h4-8,11,16,28H,9-10H2,1-3H3,(H,25,26)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 489 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Antibacterial activity against Escherichia coli DNA gyrase |
J Med Chem 44: 619-26 (2001)
Article DOI: 10.1021/jm0010623
BindingDB Entry DOI: 10.7270/Q2T43WT3 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50471900

(CHEMBL335700)Show SMILES N[C@H]1CCN(C1)c1c(F)cc2c3c1oc1c4ccccc4ccc1n3cc(C(O)=O)c2=O |r| Show InChI InChI=1S/C24H18FN3O4/c25-17-9-15-19-23(20(17)27-8-7-13(26)10-27)32-22-14-4-2-1-3-12(14)5-6-18(22)28(19)11-16(21(15)29)24(30)31/h1-6,9,11,13H,7-8,10,26H2,(H,30,31)/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase II as conversion of catenated to decatenated KDNA |
J Med Chem 41: 4273-8 (1998)
Article DOI: 10.1021/jm980265c
BindingDB Entry DOI: 10.7270/Q25X2CPR |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50470684

(CHEMBL173975)Show SMILES Cl.Cl.C1CNC(=NC1)c1ccc(cc1)-c1ccc(o1)-c1ccc(cc1)C1=NCCCN1 |c:3,t:27| Show InChI InChI=1S/C24H24N4O/c1-13-25-23(26-14-1)19-7-3-17(4-8-19)21-11-12-22(29-21)18-5-9-20(10-6-18)24-27-15-2-16-28-24/h3-12H,1-2,13-16H2,(H,25,26)(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibitory activity for 50% on topoisomerase II isolated from Giardia lamblia |
J Med Chem 38: 912-6 (1995)
Article DOI: 10.1021/jm00006a009
BindingDB Entry DOI: 10.7270/Q2NC63ZC |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50470690
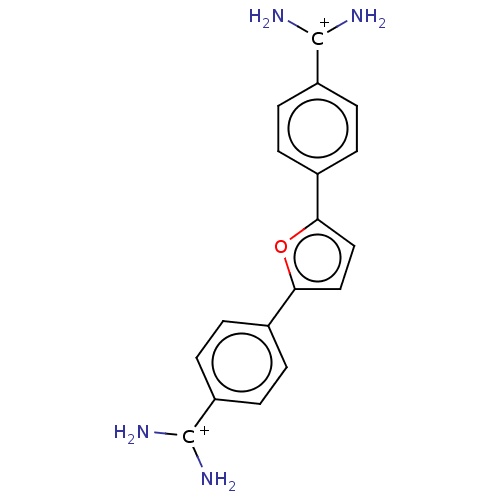
(CHEMBL367883)Show SMILES [Cl-].[Cl-].N[C+](N)c1ccc(cc1)-c1ccc(o1)-c1ccc(cc1)[C+](N)N Show InChI InChI=1S/C18H16N4O/c19-17(20)13-5-1-11(2-6-13)15-9-10-16(23-15)12-3-7-14(8-4-12)18(21)22/h1-10H,(H3,19,20)(H3,21,22)/p+2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibitory activity for 50% on topoisomerase II isolated from Giardia lamblia |
J Med Chem 38: 912-6 (1995)
Article DOI: 10.1021/jm00006a009
BindingDB Entry DOI: 10.7270/Q2NC63ZC |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50471895

(A-62176 | CHEMBL101299)Show SMILES N[C@H]1CCN(C1)c1c(F)cc2c3c1oc1ccccc1n3cc(C(O)=O)c2=O |r| Show InChI InChI=1S/C20H16FN3O4/c21-13-7-11-16-19(17(13)23-6-5-10(22)8-23)28-15-4-2-1-3-14(15)24(16)9-12(18(11)25)20(26)27/h1-4,7,9-10H,5-6,8,22H2,(H,26,27)/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin
Curated by ChEMBL
| Assay Description
Inhibition of topoisomerase II as conversion of catenated to decatenated KDNA |
J Med Chem 41: 4273-8 (1998)
Article DOI: 10.1021/jm980265c
BindingDB Entry DOI: 10.7270/Q25X2CPR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data