

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50066430 2-(4-Methyl-piperazin-1-yl)-5-(2,3,5-trichloro-phenyl)-pyrimidin-4-ylamine::2-(4-Methyl-piperazin-1-yl)-5-(2,3,5-trichloro-phenyl)-pyrimidin-4-ylamine(BW619C89)::CHEMBL28854::SIPATRIGINE
SMILES: CN1CCN(CC1)c1ncc(c(N)n1)-c1cc(Cl)cc(Cl)c1Cl
InChI Key: InChIKey=PDOCBJADCWMDGL-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium channel protein type I I alpha subunit (Rattus norvegicus) | BDBM50066430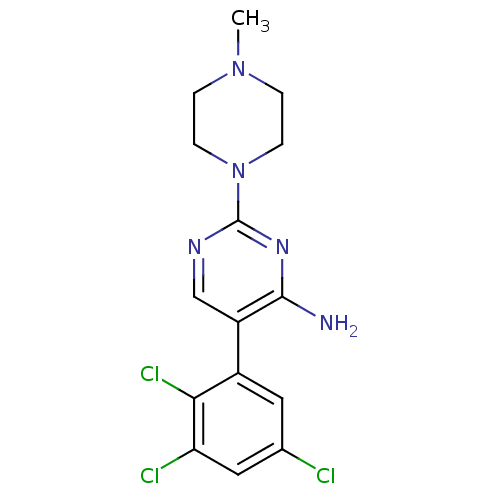 (2-(4-Methyl-piperazin-1-yl)-5-(2,3,5-trichloro-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma KG Curated by ChEMBL | Assay Description Displacement of [3H]BTX from sodium channel of rat cerebral cortex synaptosomes | J Med Chem 45: 3755-64 (2002) Article DOI: 10.1021/jm020875j BindingDB Entry DOI: 10.7270/Q2ZS308Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 alpha subunit (Homo sapiens (Human)) | BDBM50066430 (2-(4-Methyl-piperazin-1-yl)-5-(2,3,5-trichloro-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents | Article | n/a | n/a | 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Blockade of synaptosomal sodium channels, inhibition of [14C]-guanidinium ion flux into CHO cells expressing Type II sodium channel | Bioorg Med Chem Lett 5: 2259-2262 (1995) Article DOI: 10.1016/0960-894X(95)00392-7 BindingDB Entry DOI: 10.7270/Q200022B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type II alpha subunit (Rattus norvegicus) | BDBM50066430 (2-(4-Methyl-piperazin-1-yl)-5-(2,3,5-trichloro-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience, Inc. Curated by ChEMBL | Assay Description Sodium channel block, determined by % block of [14C]guanidinium flux in CHO line expressing rat type IIA sodium channels derived from rat brain | J Med Chem 41: 3298-302 (1998) Article DOI: 10.1021/jm980134b BindingDB Entry DOI: 10.7270/Q2Q52Q8W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type I I alpha subunit (Rattus norvegicus) | BDBM50066430 (2-(4-Methyl-piperazin-1-yl)-5-(2,3,5-trichloro-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma KG Curated by ChEMBL | Assay Description Inhibitory effect against veratridine-induced glutamate release from rat brain slices | J Med Chem 45: 3755-64 (2002) Article DOI: 10.1021/jm020875j BindingDB Entry DOI: 10.7270/Q2ZS308Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Twik-RElated Potassium (K+) channel 1 (TREK1) (Homo sapiens (Human)) | BDBM50066430 (2-(4-Methyl-piperazin-1-yl)-5-(2,3,5-trichloro-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | 7.3 | 22 |
Korea Institute of Science and Technology | Assay Description The hTREK1 stable cell lines were seeded at a density of 10 000 cells/well in a 12-well plate. Whole-cell membrane currents were amplified using the ... | Chem Biol Drug Des 88: 807-819 (2016) Article DOI: 10.1111/cbdd.12810 BindingDB Entry DOI: 10.7270/Q2S181B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Twik-RElated Potassium (K+) channel 1 (TREK1) (Homo sapiens (Human)) | BDBM50066430 (2-(4-Methyl-piperazin-1-yl)-5-(2,3,5-trichloro-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Clermont Auvergne Curated by ChEMBL | Assay Description Inhibition of of human TREK1 expressed in HEK293 cells assessed as reversible current depression | J Med Chem 59: 5149-57 (2016) BindingDB Entry DOI: 10.7270/Q2319XSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||