

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: COc1cc(OC)c(F)c(c1F)-c1ccc(C(=O)Nc2ccc(CN3CCN(C)CC3)cn2)c2ncccc12
InChI Key: InChIKey=LYMUUHRXBLNABW-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM102548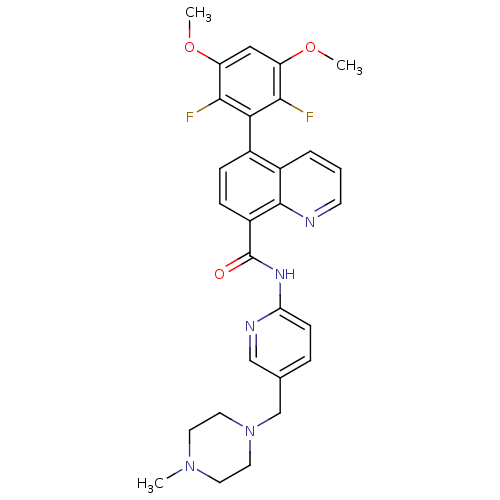 (US8536175, 174) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis AG US Patent | Assay Description The assay has been run at room temperature on a liquid handling robot. To the assay plates containing 50 mL compound or control solutions, 4.5 uL of ... | US Patent US8815901 (2014) BindingDB Entry DOI: 10.7270/Q2XG9PT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM102548 (US8536175, 174) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Protein kinase activity measured by the microfluidic caliper method. | US Patent US8536175 (2013) BindingDB Entry DOI: 10.7270/Q27D2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||