

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM10295 4-nitro-N-(1,3,4-trioxo-1,2,3,4-tetrahydroisoquinolin-7-yl)benzamide::Isoquinoline-1,3,4-trione 14c::N-(1,2,3,4-Tetrahydro-1,3,4-trioxoisoquinolin-7-yl)-4-nitrobenzamide
SMILES: O=C(Nc1ccc2C(=O)C(=O)NC(=O)c2c1)c1ccc(cc1)N(=O)=O
InChI Key: InChIKey=NGYGGBLAVBSGBJ-UHFFFAOYSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caspase-3 (Homo sapiens (Human)) | BDBM10295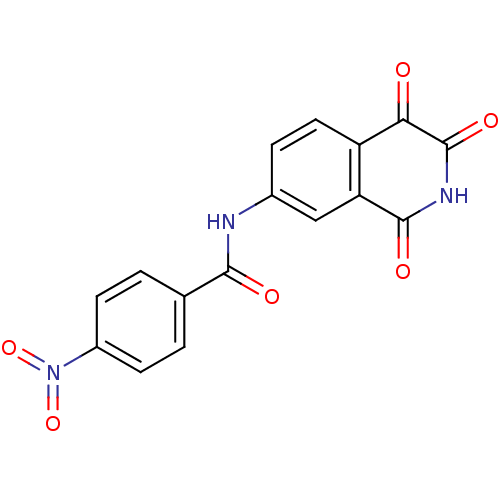 (4-nitro-N-(1,3,4-trioxo-1,2,3,4-tetrahydroisoquino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institutes for Biological Sciences | Assay Description The rate of chromogenic substrate hydrolysis was monitored by the change of absorbance at 405 nm for 3 min. Compounds were tested in duplicate. The I... | J Med Chem 49: 1613-23 (2006) Article DOI: 10.1021/jm050896o BindingDB Entry DOI: 10.7270/Q23N21MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM10295 (4-nitro-N-(1,3,4-trioxo-1,2,3,4-tetrahydroisoquino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institutes for Biological Sciences | Assay Description The rate of chromogenic substrate hydrolysis was monitored by the change of absorbance at 405 nm for 3 min. Compounds were tested in duplicate. The I... | J Med Chem 49: 1613-23 (2006) Article DOI: 10.1021/jm050896o BindingDB Entry DOI: 10.7270/Q23N21MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-8 (Homo sapiens (Human)) | BDBM10295 (4-nitro-N-(1,3,4-trioxo-1,2,3,4-tetrahydroisoquino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institutes for Biological Sciences | Assay Description The rate of chromogenic substrate hydrolysis was monitored by the change of absorbance at 405 nm for 3 min. Compounds were tested in duplicate. The I... | J Med Chem 49: 1613-23 (2006) Article DOI: 10.1021/jm050896o BindingDB Entry DOI: 10.7270/Q23N21MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-7 (Homo sapiens (Human)) | BDBM10295 (4-nitro-N-(1,3,4-trioxo-1,2,3,4-tetrahydroisoquino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institutes for Biological Sciences | Assay Description The rate of chromogenic substrate hydrolysis was monitored by the change of absorbance at 405 nm for 3 min. Compounds were tested in duplicate. The I... | J Med Chem 49: 1613-23 (2006) Article DOI: 10.1021/jm050896o BindingDB Entry DOI: 10.7270/Q23N21MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-6 (Homo sapiens (Human)) | BDBM10295 (4-nitro-N-(1,3,4-trioxo-1,2,3,4-tetrahydroisoquino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institutes for Biological Sciences | Assay Description The rate of chromogenic substrate hydrolysis was monitored by the change of absorbance at 405 nm for 3 min. Compounds were tested in duplicate. The I... | J Med Chem 49: 1613-23 (2006) Article DOI: 10.1021/jm050896o BindingDB Entry DOI: 10.7270/Q23N21MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||