

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM10583 3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{6-[(1,2,3,4-tetrahydroacridin-9-yl)amino]hexyl}amino}-7,11-methanocycloocta[b]quinoline dihydrochloride::7-chloro-15-methyl-N-[6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexyl]-10-azatetracyclo[11.3.1.0^{2,11}.0^{4,9}]heptadeca-2(11),3,5,7,9,14-hexaen-3-amine dihydrochloride::CHEMBL2011483::Huprine-Tacrine Heterodimer 17a
SMILES: CC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1NCCCCCCNc1c2CCCCc2nc2ccccc12
InChI Key: InChIKey=HLRVNJVVXBHFTE-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10583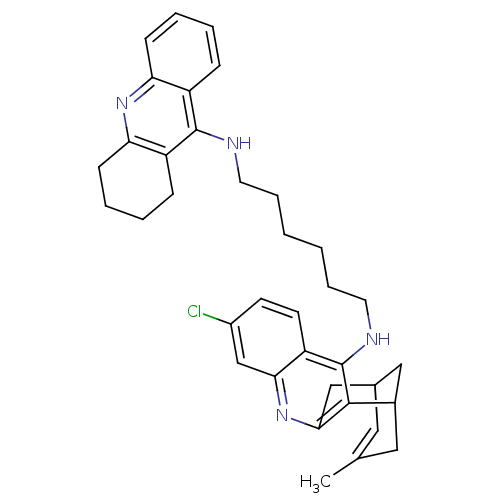 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{6-[(1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 48: 1701-4 (2005) Article DOI: 10.1021/jm0496741 BindingDB Entry DOI: 10.7270/Q21N7ZB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases (Homo sapiens (Human)) | BDBM10583 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{6-[(1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 mins b... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10583 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{6-[(1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases (Homo sapiens (Human)) | BDBM10583 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{6-[(1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.69 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | J Med Chem 48: 1701-4 (2005) Article DOI: 10.1021/jm0496741 BindingDB Entry DOI: 10.7270/Q21N7ZB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||