

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM106857 US8592455, 57
SMILES: C[C@@H]1C[C@H](N)C[C@@H](C1)c1ccncc1NC(=O)c1ccc(F)c(n1)-c1ccccc1F
InChI Key: InChIKey=ATNCLUHEGCYVPT-XHSDSOJGSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM106857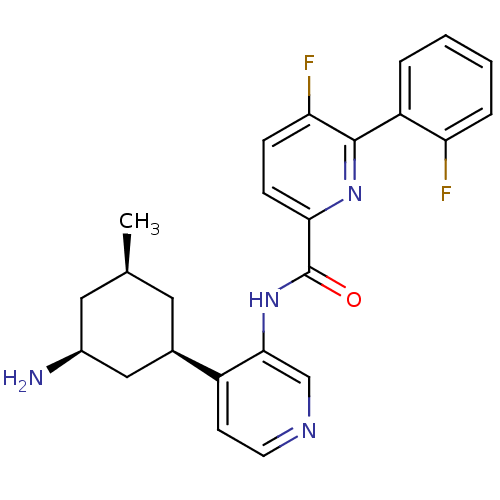 (US8592455, 57) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 kinase (unknown origin) using NH2-AGAGRSRHSSYPAGT-OH as substrate by kinase-Glo assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM106857 (US8592455, 57) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM3 kinase (unknown origin) using NH2-AGAGRSRHSSYPAGT-OH as substrate by kinase-Glo assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM106857 (US8592455, 57) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM2 kinase (unknown origin) using NH2-AGAGRSRHSSYPAGT-OH as substrate by kinase-Glo assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM106857 (US8592455, 57) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG US Patent | Assay Description The activity of PIM1, PIM2, and PIM3 is measured using a luciferase-luciferin based ATP detection reagent to quantify ATP depletion resulting from ki... | US Patent US8592455 (2013) BindingDB Entry DOI: 10.7270/Q2FF3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM106857 (US8592455, 57) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG US Patent | Assay Description The activity of PIM1, PIM2, and PIM3 is measured using a luciferase-luciferin based ATP detection reagent to quantify ATP depletion resulting from ki... | US Patent US8592455 (2013) BindingDB Entry DOI: 10.7270/Q2FF3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM106857 (US8592455, 57) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG US Patent | Assay Description The activity of PIM1, PIM2, and PIM3 is measured using a luciferase-luciferin based ATP detection reagent to quantify ATP depletion resulting from ki... | US Patent US8592455 (2013) BindingDB Entry DOI: 10.7270/Q2FF3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||