

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM107604 US8575157, 53
SMILES: CCn1cc(ccc1=O)-c1ccc(cc1)[C@H](C)N1CC[C@@](CC(C)(C)O)(OC1=O)c1ccccc1
InChI Key: InChIKey=ZABDLBFFXBIVLQ-LGGPFLRQSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107604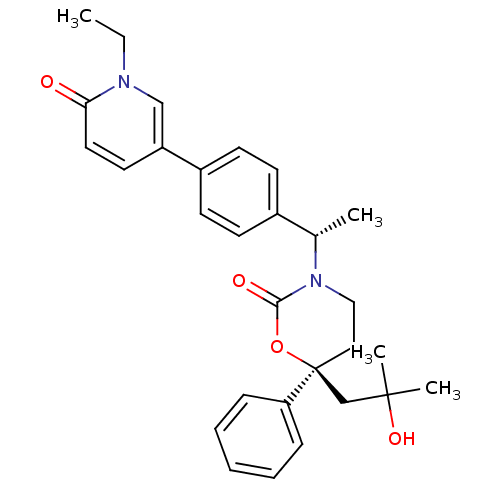 (US8575157, 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.35 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The inhibition of a microsomal preparation of 11β-HSD1 by compounds of the invention was measured essentially as previously described (K. Solly,... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM107604 (US8575157, 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.19 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Inhibition assay using 11β-HSD1 in the presence of 50% human plasma. | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM107604 (US8575157, 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The inhibition of recombinant CYP2C9 by compounds of the invention was measured using a commercial kit from Invitrogen (cat #2859). | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM107604 (US8575157, 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C9-isoenzyme catalysed O-demethylation o... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM107604 (US8575157, 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description Using a procedure similar to that described in Biological Test Example 6, the inhibition of cytochrome P450 2C19-isoenzyme catalysed N-demethylation ... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM107604 (US8575157, 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Vitae Pharmaceuticals, Inc.; Boehringer Ingelheim International, GmbH US Patent | Assay Description The assay was based on a method published by Moody et al. (Xenobiotica 1999). The inhibition of cytochrome P450 3A4-isoenzyme catalysed N-demethylati... | US Patent US8575157 (2013) BindingDB Entry DOI: 10.7270/Q2416VPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||