

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM108439 US8609688, 17
SMILES: C[C@H]1CN(C[C@@H](C)O1)c1cccc(c1)-c1ccc2nc(-c3cccnc3N)n(-c3ccc(cc3)C3(N)CCC3)c2n1
InChI Key: InChIKey=LZWGJFSNUBMYRN-SZPZYZBQSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108439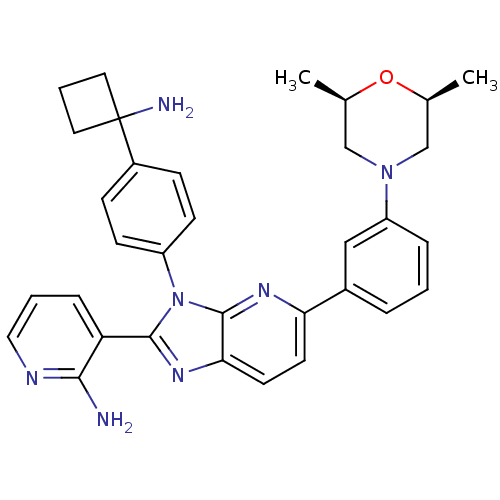 (US8609688, 17) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 3.10 | n/a | n/a | n/a | 7.5 | 80 |
ArQule, Inc. US Patent | Assay Description Protein-ligand binding was identified with the thermal shift assay which is based on the ligand-induced stabilization of the protein tertiary structu... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108439 (US8609688, 17) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.36 | n/a | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108439 (US8609688, 17) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a |
ArQule, Inc. US Patent | Assay Description Binding analysis. Protein-ligand binding was identified with the thermal shift assay which is based on the ligand-induced stabilization of the protei... | US Patent US8962619 (2015) BindingDB Entry DOI: 10.7270/Q2W957W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM108439 (US8609688, 17) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.36 | n/a | n/a | n/a | n/a | 8.0 | 25 |
ArQule, Inc. US Patent | Assay Description AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum... | US Patent US8609688 (2013) BindingDB Entry DOI: 10.7270/Q2H41Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||