

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM10997 (1R)-2,3-dihydro-1H-inden-1-amine::1(R)-aminoindan::R-AI::rasagiline analog
SMILES: N[C@@H]1CCc2ccccc12
InChI Key: InChIKey=XJEVHMGJSYVQBQ-SECBINFHSA-N
Data: 2 KI
PDB links: 34 PDB IDs contain this monomer as substructures. 39 PDB IDs contain inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM10997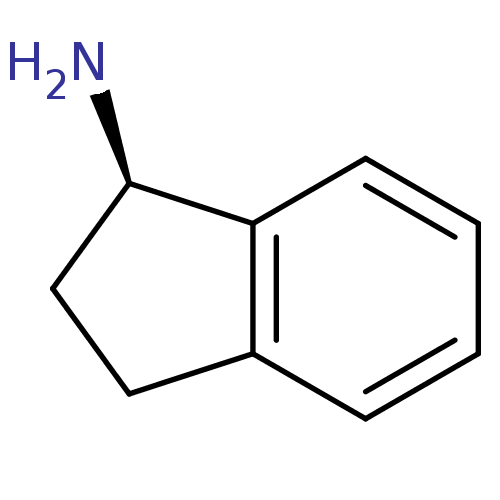 ((1R)-2,3-dihydro-1H-inden-1-amine | 1(R)-aminoinda...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.20E+4 | -6.13 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Pavia | Assay Description MAO A and MAO B activities were determined spectrophotometrically at 316 nm and 250 nm using kynuramine and benzylamine as substrates, respectively. ... | J Med Chem 48: 8148-54 (2005) Article DOI: 10.1021/jm0506266 BindingDB Entry DOI: 10.7270/Q2T151WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase (flavin-containing) A (Homo sapiens (Human)) | BDBM10997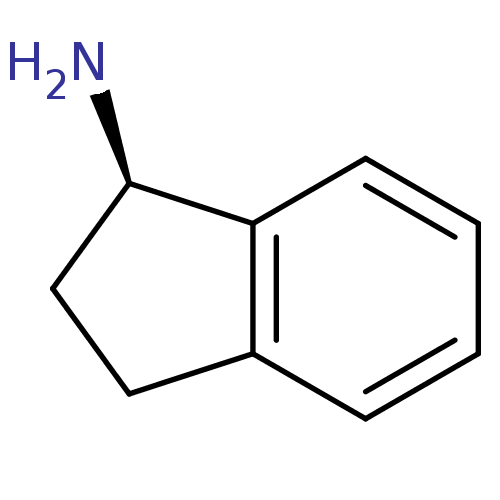 ((1R)-2,3-dihydro-1H-inden-1-amine | 1(R)-aminoinda...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 6.70E+4 | -5.69 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Pavia | Assay Description MAO A and MAO B activities were determined spectrophotometrically at 316 nm and 250 nm using kynuramine and benzylamine as substrates, respectively. ... | J Med Chem 48: 8148-54 (2005) Article DOI: 10.1021/jm0506266 BindingDB Entry DOI: 10.7270/Q2T151WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||