

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM11316 1,8-dihydroxy-9,10-dihydroanthracene-9,10-dione::1,8-dihydroxy-anthraquinone::Anthraquinone-related compound::CHEMBL53418
SMILES: Oc1cccc2C(=O)c3cccc(O)c3C(=O)c12
InChI Key: InChIKey=QBPFLULOKWLNNW-UHFFFAOYSA-N
PDB links: 1 PDB ID matches this monomer. 62 PDB IDs contain this monomer as substructures. 62 PDB IDs contain inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Casein Kinase II (Zea mays (Maize)) | BDBM11316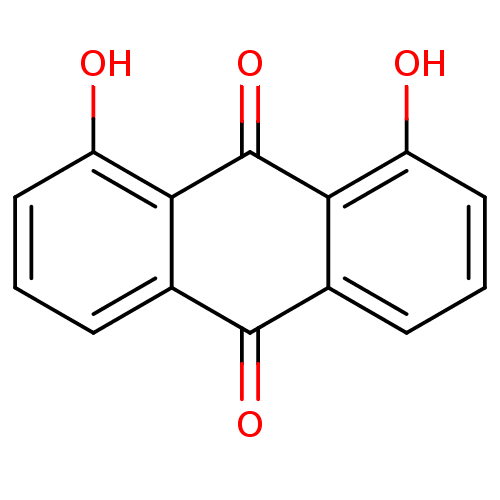 (1,8-dihydroxy-9,10-dihydroanthracene-9,10-dione | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >4.00E+4 | >-5.94 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Padova | Assay Description In vitro kinase assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of 100 uM ATP/ [gam... | J Biol Chem 278: 1831-6 (2003) Article DOI: 10.1074/jbc.M209367200 BindingDB Entry DOI: 10.7270/Q2GH9G5K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thioredoxin reductase 2, mitochondrial (Rattus norvegicus) | BDBM11316 (1,8-dihydroxy-9,10-dihydroanthracene-9,10-dione | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of rat liver mitochondrial TrxR2 by spectrophotometry | Bioorg Med Chem 19: 631-41 (2011) Article DOI: 10.1016/j.bmc.2010.10.045 BindingDB Entry DOI: 10.7270/Q25D8S4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thioredoxin reductase 1, cytoplasmic (Rattus norvegicus) | BDBM11316 (1,8-dihydroxy-9,10-dihydroanthracene-9,10-dione | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Inhibition of rat liver cytosolic TrxR1 by spectrophotometry | Bioorg Med Chem 19: 631-41 (2011) Article DOI: 10.1016/j.bmc.2010.10.045 BindingDB Entry DOI: 10.7270/Q25D8S4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase (Bos taurus) | BDBM11316 (1,8-dihydroxy-9,10-dihydroanthracene-9,10-dione | ...) | PDB MMDB Reactome pathway UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universität Münster Curated by ChEMBL | Assay Description 5-LO inhibitory activity was determined by inhibition of LTB4 biosynthesis in bovine polymorphonuclear leukocytes (PMNL) | J Med Chem 40: 3773-80 (1997) Article DOI: 10.1021/jm970292n BindingDB Entry DOI: 10.7270/Q20R9NJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase 2 (Homo sapiens (Human)) | BDBM11316 (1,8-dihydroxy-9,10-dihydroanthracene-9,10-dione | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of human Neu2 assessed as MuNANA substrate hydrolysis in presence of 0.1% Triton X-100 by discontinuous fluorimetric assay | Bioorg Med Chem 18: 1633-40 (2010) Article DOI: 10.1016/j.bmc.2009.12.062 BindingDB Entry DOI: 10.7270/Q26110F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||