

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM11338 Hydroxamate 19::N-hydroxy-3-methyl-2-[(1,1,2,2,3,3,4,4,4-nonafluorobutane)sulfonamido]butanamide
SMILES: CC(C)C(NS(=O)(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F)C(=O)NO
InChI Key: InChIKey=JCUTWTFOYUXMPS-UHFFFAOYSA-N
Data: 10 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11338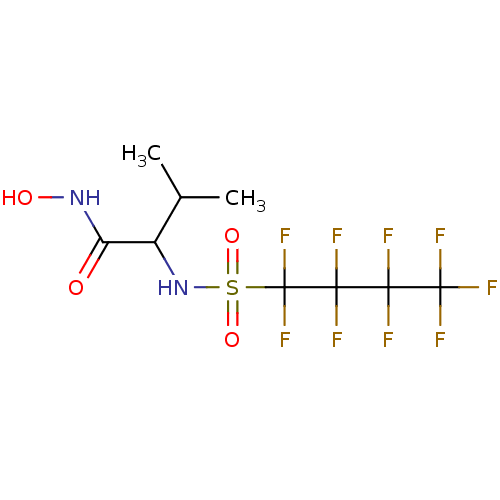 (Hydroxamate 19 | N-hydroxy-3-methyl-2-[(1,1,2,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 43: 3677-87 (2000) Article DOI: 10.1021/jm000027t BindingDB Entry DOI: 10.7270/Q2736P45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic Anhydrase IV (Bos taurus (bovine)) | BDBM11338 (Hydroxamate 19 | N-hydroxy-3-methyl-2-[(1,1,2,2,3,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 43: 3677-87 (2000) Article DOI: 10.1021/jm000027t BindingDB Entry DOI: 10.7270/Q2736P45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM11338 (Hydroxamate 19 | N-hydroxy-3-methyl-2-[(1,1,2,2,3,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 29 | -10.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 43: 3677-87 (2000) Article DOI: 10.1021/jm000027t BindingDB Entry DOI: 10.7270/Q2736P45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM11338 (Hydroxamate 19 | N-hydroxy-3-methyl-2-[(1,1,2,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pomona College Curated by ChEMBL | Assay Description Inhibition of MMP2 | Bioorg Med Chem 15: 2223-68 (2007) Article DOI: 10.1016/j.bmc.2007.01.011 BindingDB Entry DOI: 10.7270/Q2571DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM11338 (Hydroxamate 19 | N-hydroxy-3-methyl-2-[(1,1,2,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi | Assay Description Initial rates for the hydrolysis of the thioester substrate AcProLeuGly-S-LeuLeuGlyOEt, coupled to the reaction with 5,5-dithiobis(2-nitrobenzoic aci... | J Med Chem 43: 3677-87 (2000) Article DOI: 10.1021/jm000027t BindingDB Entry DOI: 10.7270/Q2736P45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase (ChC) (Clostridium histolyticum) | BDBM11338 (Hydroxamate 19 | N-hydroxy-3-methyl-2-[(1,1,2,2,3,...) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi | Assay Description The rate of hydrolysis was determined from the change in absorbance at 324 nm using an extinction coefficient, 24700 M-1 cm-1 for FALGPA. Initial vel... | J Med Chem 43: 3677-87 (2000) Article DOI: 10.1021/jm000027t BindingDB Entry DOI: 10.7270/Q2736P45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM11338 (Hydroxamate 19 | N-hydroxy-3-methyl-2-[(1,1,2,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi | Assay Description Initial rates for the hydrolysis of the thioester substrate AcProLeuGly-S-LeuLeuGlyOEt, coupled to the reaction with 5,5-dithiobis(2-nitrobenzoic aci... | J Med Chem 43: 3677-87 (2000) Article DOI: 10.1021/jm000027t BindingDB Entry DOI: 10.7270/Q2736P45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM11338 (Hydroxamate 19 | N-hydroxy-3-methyl-2-[(1,1,2,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi | Assay Description Initial rates for the hydrolysis of the thioester substrate AcProLeuGly-S-LeuLeuGlyOEt, coupled to the reaction with 5,5-dithiobis(2-nitrobenzoic aci... | J Med Chem 43: 3677-87 (2000) Article DOI: 10.1021/jm000027t BindingDB Entry DOI: 10.7270/Q2736P45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM11338 (Hydroxamate 19 | N-hydroxy-3-methyl-2-[(1,1,2,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pomona College Curated by ChEMBL | Assay Description Inhibition of MMP9 | Bioorg Med Chem 15: 2223-68 (2007) Article DOI: 10.1016/j.bmc.2007.01.011 BindingDB Entry DOI: 10.7270/Q2571DBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM11338 (Hydroxamate 19 | N-hydroxy-3-methyl-2-[(1,1,2,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi | Assay Description Initial rates for the hydrolysis of the thioester substrate AcProLeuGly-S-LeuLeuGlyOEt, coupled to the reaction with 5,5-dithiobis(2-nitrobenzoic aci... | J Med Chem 43: 3677-87 (2000) Article DOI: 10.1021/jm000027t BindingDB Entry DOI: 10.7270/Q2736P45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||