

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM11525 (1S,3S,5S)-2-[(2S)-2-amino-3,3-dimethylpent-4-enoyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2,2,2-trifluoroacetic acid::(S)-2-(1,1-Dimethylprop-2-en-1-yl)glycine-L-cis-4,5-methanoprolinenitrile TFA salt::BMS-477118 analogue::Saxagliptin Analogue 8a
SMILES: CC(C)(C=C)[C@H](N)C(=O)N1[C@H]2C[C@H]2C[C@H]1C#N
InChI Key: InChIKey=GSCSWPJNZSKJNO-VPOLOUISSA-N
Data: 1 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11525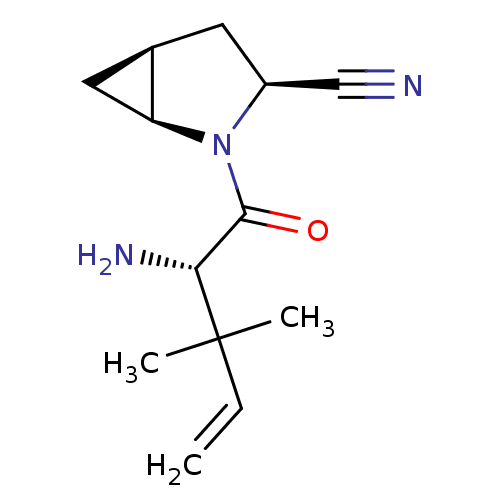 ((1S,3S,5S)-2-[(2S)-2-amino-3,3-dimethylpent-4-enoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 57 | -9.78 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||