

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM118011 CHEMBL590503::US8637558, 7
SMILES: OC1=NC(=O)C(S1)=Cc1ccc(OCCNC2CCCCC2)cc1
InChI Key: InChIKey=QJGMTHLUFPOPPA-UHFFFAOYSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15-hydroxyprostaglandin dehydrogenase [NAD+] (Homo sapiens (Human)) | BDBM118011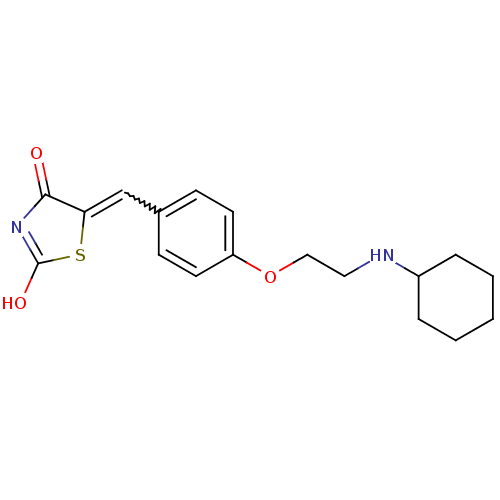 (CHEMBL590503 | US8637558, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human 15PGDH expressed in Escherichia coli BL-21 by fluorescence spectrophotometry | Bioorg Med Chem 18: 1428-33 (2010) Article DOI: 10.1016/j.bmc.2010.01.016 BindingDB Entry DOI: 10.7270/Q2Z0393H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD+] (Homo sapiens (Human)) | BDBM118011 (CHEMBL590503 | US8637558, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.61E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||