

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM11994 2-{[4-(2,5-dimethyl-1H-pyrrol-1-yl)phenyl]sulfanyl}acetic acid::Interchim compound 56
SMILES: Cc1ccc(C)n1-c1ccc(SCC(O)=O)cc1
InChI Key: InChIKey=DASVXRJADIYRNJ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anthrax Lethal Factor (LF) (Bacillus anthracis) | BDBM11994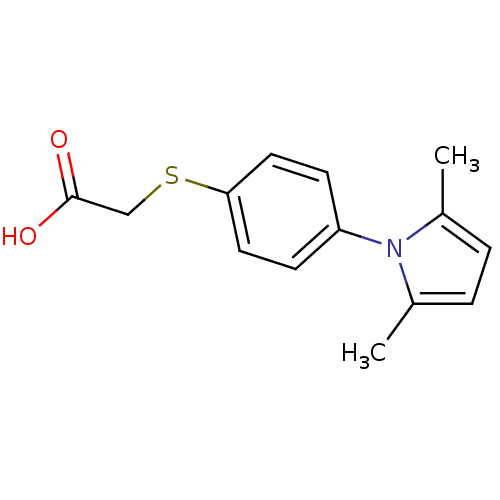 (2-{[4-(2,5-dimethyl-1H-pyrrol-1-yl)phenyl]sulfanyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | 1.60E+3 | -7.95 | 2.90E+3 | n/a | n/a | n/a | n/a | 7.2 | 27 |
Montana State University | Assay Description For selected lead compounds from fluorescence-based high-throughput screening, the concentrations of inhibitor that caused 50% inhibition of enzymati... | J Med Chem 49: 5232-44 (2006) Article DOI: 10.1021/jm0605132 BindingDB Entry DOI: 10.7270/Q20C4T19 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase (VHR) (Homo sapiens (Human)) | BDBM11994 (2-{[4-(2,5-dimethyl-1H-pyrrol-1-yl)phenyl]sulfanyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoid receptor (Homo sapiens (Human)) | BDBM11994 (2-{[4-(2,5-dimethyl-1H-pyrrol-1-yl)phenyl]sulfanyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Swiss Federal Institute of Technology (ETH) Curated by ChEMBL | Assay Description Transactivation of human Gal4-fused RXRbeta LBD expressed in HEK293T cells after 12 to 14 hrs by dual-glo luciferase assay | J Med Chem 61: 5442-5447 (2018) Article DOI: 10.1021/acs.jmedchem.8b00494 BindingDB Entry DOI: 10.7270/Q2J38W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha/gamma (Homo sapiens (Human)) | BDBM11994 (2-{[4-(2,5-dimethyl-1H-pyrrol-1-yl)phenyl]sulfanyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Swiss Federal Institute of Technology (ETH) Curated by ChEMBL | Assay Description Transactivation of human Gal4-fused RXRalpha LBD expressed in HEK293T cells after 12 to 14 hrs by dual-glo luciferase assay | J Med Chem 61: 5442-5447 (2018) Article DOI: 10.1021/acs.jmedchem.8b00494 BindingDB Entry DOI: 10.7270/Q2J38W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DnaC helicase (Staphylococcus aureus) | BDBM11994 (2-{[4-(2,5-dimethyl-1H-pyrrol-1-yl)phenyl]sulfanyl...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus Smith DNA helicase DnaC assessed as strand unwinding after 30 mins by FRET assay | Bioorg Med Chem 17: 4466-76 (2009) Article DOI: 10.1016/j.bmc.2009.05.014 BindingDB Entry DOI: 10.7270/Q22F7NGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||