

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM127661 US8791123, 34
SMILES: OCc1ccc(F)cc1C1CCCN1c1ccn2ncc(C(=O)NC3CCC(O)CC3)c2n1
InChI Key: InChIKey=RJWUXXDSPBTHTI-UHFFFAOYSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM127661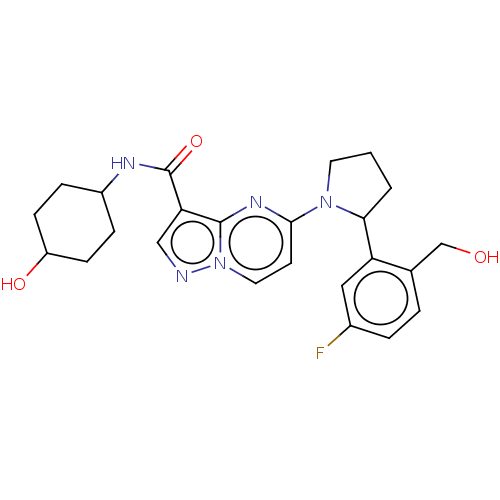 (US8791123, 34) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 42.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Array Biopharma, Inc. US Patent | Assay Description Compounds of Formula I were screened for their ability to inhibit Jak2 using the general enzyme inhibition assay method, in which the assay mixture c... | US Patent US8791123 (2014) BindingDB Entry DOI: 10.7270/Q2Q23XX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM127661 (US8791123, 34) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Array Biopharma, Inc. US Patent | Assay Description An enzyme-linked immunosorbant assay (ELISA) was used to assess TrkA kinase activity in the presence of inhibitors. Immulon 4HBX 384-well microtiter ... | US Patent US8791123 (2014) BindingDB Entry DOI: 10.7270/Q2Q23XX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||