

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM12975 (2S)-2-[(2S)-2-({[(1R)-1-(4-bromophenyl)ethyl]carbamoyl}amino)-3-(1H-indol-3-yl)propanamido]-N-[(2S)-5-carbamimidamido-1-oxo-1-(1,3-thiazol-2-yl)pentan-2-yl]-3-methylbutanamide::alpha-ketothiazole analogue 35
SMILES: CC(C)[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)N[C@H](C)c1ccc(Br)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)c1nccs1
InChI Key: InChIKey=RXTVQIZDVTYZLH-DLMOMKDTSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor XI (Homo sapiens (Human)) | BDBM12975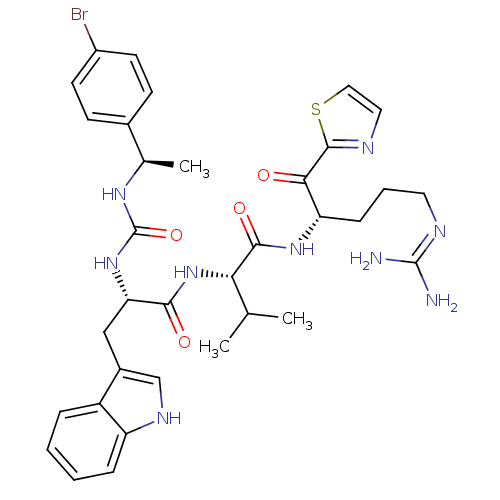 ((2S)-2-[(2S)-2-({[(1R)-1-(4-bromophenyl)ethyl]carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED) | Assay Description Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... | J Med Chem 49: 7781-91 (2006) Article DOI: 10.1021/jm060978s BindingDB Entry DOI: 10.7270/Q2SJ1HTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM12975 ((2S)-2-[(2S)-2-({[(1R)-1-(4-bromophenyl)ethyl]carb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED) | Assay Description Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... | J Med Chem 49: 7781-91 (2006) Article DOI: 10.1021/jm060978s BindingDB Entry DOI: 10.7270/Q2SJ1HTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12975 ((2S)-2-[(2S)-2-({[(1R)-1-(4-bromophenyl)ethyl]carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED) | Assay Description Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... | J Med Chem 49: 7781-91 (2006) Article DOI: 10.1021/jm060978s BindingDB Entry DOI: 10.7270/Q2SJ1HTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||