

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM14057 N-[(3S)-1-(4,7-diazabicyclo[4.3.0]nona-2,4,8,10-tetraen-8-ylmethyl)-2-oxo-pyrrolidin-3-yl]-7-thia-2-azabicyclo[4.3.0]nona-2,4,8,10-tetraene-8-sulfonamide::N-[(3S)-2-oxo-1-{1H-pyrrolo[2,3-c]pyridin-2-ylmethyl}pyrrolidin-3-yl]thieno[3,2-b]pyridine-2-sulfonamide::RPR208707
SMILES: O=C1[C@H](CCN1Cc1cc2ccncc2[nH]1)NS(=O)(=O)c1cc2ncccc2s1
InChI Key: InChIKey=PLXOQMHGHDZMSX-AWEZNQCLSA-N
Data: 4 KI
PDB links: 1 PDB ID matches this monomer. 1 PDB ID contains inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM14057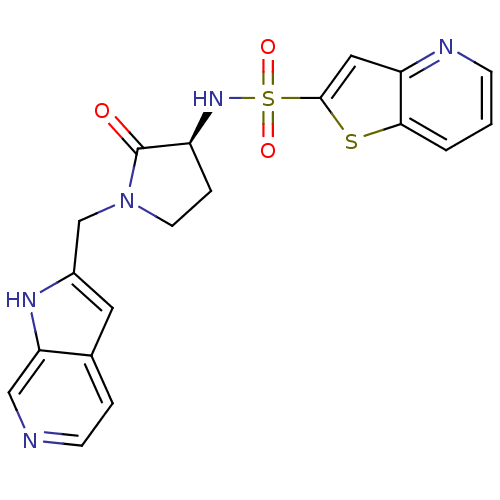 (N-[(3S)-1-(4,7-diazabicyclo[4.3.0]nona-2,4,8,10-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 18 | -10.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Aventis Pharma | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 43: 3226-32 (2000) Article DOI: 10.1021/jm000940u BindingDB Entry DOI: 10.7270/Q25H7DHP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM14057 (N-[(3S)-1-(4,7-diazabicyclo[4.3.0]nona-2,4,8,10-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of factor 10a | J Med Chem 53: 6243-74 (2010) Article DOI: 10.1021/jm100146h BindingDB Entry DOI: 10.7270/Q2CR5VBB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trypsin (Bos taurus (bovine)) | BDBM14057 (N-[(3S)-1-(4,7-diazabicyclo[4.3.0]nona-2,4,8,10-te...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >2.90E+3 | >-7.47 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Aventis Pharma | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 43: 3226-32 (2000) Article DOI: 10.1021/jm000940u BindingDB Entry DOI: 10.7270/Q25H7DHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14057 (N-[(3S)-1-(4,7-diazabicyclo[4.3.0]nona-2,4,8,10-te...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >4.00E+3 | >-7.28 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Aventis Pharma | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | J Med Chem 43: 3226-32 (2000) Article DOI: 10.1021/jm000940u BindingDB Entry DOI: 10.7270/Q25H7DHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||