

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM141379 US8916600, 13::US9738632, Example 13
SMILES: C[C@H](NC(=O)C(C)(F)F)[C@H](Oc1ccc2n(ncc2c1)-c1cccc(c1)C(=O)NCc1cccnc1)c1ccc2COCOc2c1
InChI Key: InChIKey=BHIQUIYSVRULQI-BGOLNKOXSA-N
Data: 11 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141379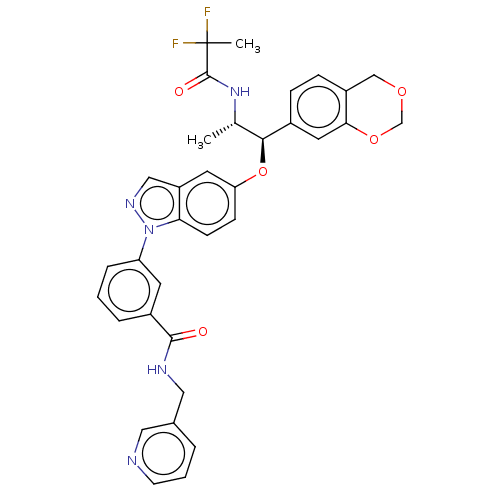 (US8916600, 13 | US9738632, Example 13) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.817 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Astrazeneca AB; Bayer Pharma Aktiengesellschaft US Patent | Assay Description In the GR radioligand binding assay, test compounds were serially diluted in semi-log steps (10 concentrations) with a final concentration of 10 uM. ... | US Patent US8916600 (2014) BindingDB Entry DOI: 10.7270/Q2WQ02HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141379 (US8916600, 13 | US9738632, Example 13) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.817 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB; Bayer Pharma Aktiengesellschaft US Patent | Assay Description In the GR radioligand binding assay, test compounds were serially diluted in semi-log steps (10 concentrations) with a final concentration of 10 _... | US Patent US9738632 (2017) BindingDB Entry DOI: 10.7270/Q2057J12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM141379 (US8916600, 13 | US9738632, Example 13) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression of glucocorticoid receptor in Wistar rat PBMC assessed as inhibition of LPS induced TNF alpha release preincubated for 45 mins follow... | J Med Chem 60: 8591-8605 (2017) Article DOI: 10.1021/acs.jmedchem.7b01215 BindingDB Entry DOI: 10.7270/Q2RX9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM141379 (US8916600, 13 | US9738632, Example 13) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone from N terminal maltose binding protein tagged human mineralocorticoid receptor LBD (726 to 984 residues) expressed ... | J Med Chem 60: 8591-8605 (2017) Article DOI: 10.1021/acs.jmedchem.7b01215 BindingDB Entry DOI: 10.7270/Q2RX9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM141379 (US8916600, 13 | US9738632, Example 13) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of fluormone labelled PL Red from human recombinant progesterone receptor after 1 to 6 hrs ligand by fluorescence polarization assay | J Med Chem 60: 8591-8605 (2017) Article DOI: 10.1021/acs.jmedchem.7b01215 BindingDB Entry DOI: 10.7270/Q2RX9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141379 (US8916600, 13 | US9738632, Example 13) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression of glucocorticoid receptor in PMA-stimulated human ChaGoK1 cells expressing TRE-LacZ construct assessed as inhibition of AP-1 mediate... | J Med Chem 60: 8591-8605 (2017) Article DOI: 10.1021/acs.jmedchem.7b01215 BindingDB Entry DOI: 10.7270/Q2RX9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM141379 (US8916600, 13 | US9738632, Example 13) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of fluormone labelled EL Red from human recombinant estrogen receptor alpha after 1 to 5 hrs by fluorescence polarization assay | J Med Chem 60: 8591-8605 (2017) Article DOI: 10.1021/acs.jmedchem.7b01215 BindingDB Entry DOI: 10.7270/Q2RX9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM141379 (US8916600, 13 | US9738632, Example 13) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of fluormone labelled EL Red from human recombinant estrogen receptor beta after 1 to 5 hrs by fluorescence polarization assay | J Med Chem 60: 8591-8605 (2017) Article DOI: 10.1021/acs.jmedchem.7b01215 BindingDB Entry DOI: 10.7270/Q2RX9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141379 (US8916600, 13 | US9738632, Example 13) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression of glucocorticoid receptor in human PBMC assessed as inhibition of LPS induced TNF alpha release preincubated for 45 mins followed by... | J Med Chem 60: 8591-8605 (2017) Article DOI: 10.1021/acs.jmedchem.7b01215 BindingDB Entry DOI: 10.7270/Q2RX9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM141379 (US8916600, 13 | US9738632, Example 13) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of fluormone labelled GS Red from human recombinant glucocorticoid receptor after 2 hrs by fluorescence polarization assay | J Med Chem 60: 8591-8605 (2017) Article DOI: 10.1021/acs.jmedchem.7b01215 BindingDB Entry DOI: 10.7270/Q2RX9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen Receptor (Rattus norvegicus (Rat)) | BDBM141379 (US8916600, 13 | US9738632, Example 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of fluormone labelled AL green from rat recombinant His/GST-tagged androgen receptor LBD after 4 to 6 hrs by fluorescence polarization a... | J Med Chem 60: 8591-8605 (2017) Article DOI: 10.1021/acs.jmedchem.7b01215 BindingDB Entry DOI: 10.7270/Q2RX9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||