

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CS(=O)(=O)NC(=O)c1cc(C2CC2)c(O[C@H]2C3CC4CC2C[C@@](Cl)(C4)C3)cc1F
InChI Key: InChIKey=IVGOGVKIRUVHNB-ZQPHQBMLSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM145578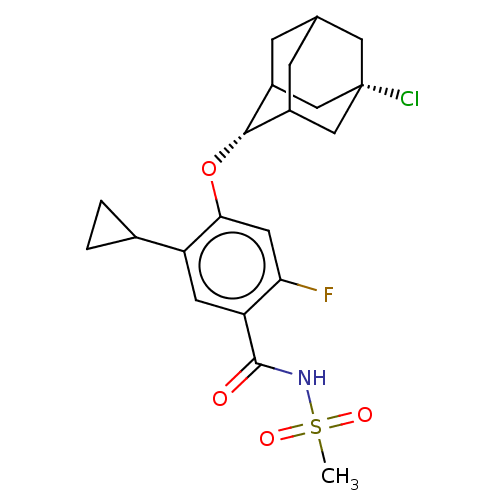 (US8952169, 375) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | 37 |
Xenon Pharmaceuticals Inc.; Genentech, Inc. US Patent | Assay Description Patch voltage clamp electrophysiology allows for the direct measurement and quantification of block of voltage-gated sodium channels (NaV's), and all... | US Patent US8952169 (2015) BindingDB Entry DOI: 10.7270/Q2M04445 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM145578 (US8952169, 375) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Xenon Pharmaceuticals Inc.; Genentech, Inc. US Patent | Assay Description Radioligand Binding Studies: Saturation experiments. A representative compound of formula (I) was tritiated. Three tritiums were incorporated in plac... | US Patent US8952169 (2015) BindingDB Entry DOI: 10.7270/Q2M04445 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||