

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM156129 US9018214, 180
SMILES: C[C@@H]1CN(CC(=O)N2CC(C)(C)c3ncc(Cc4ccccc4)cc23)[C@@H](CN2CCCC2=O)CN1
InChI Key: InChIKey=ALAFRPAHGDJVFE-NFBKMPQASA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X-linked inhibitor of apoptosis protein (XIAP) (Homo sapiens (Human)) | BDBM156129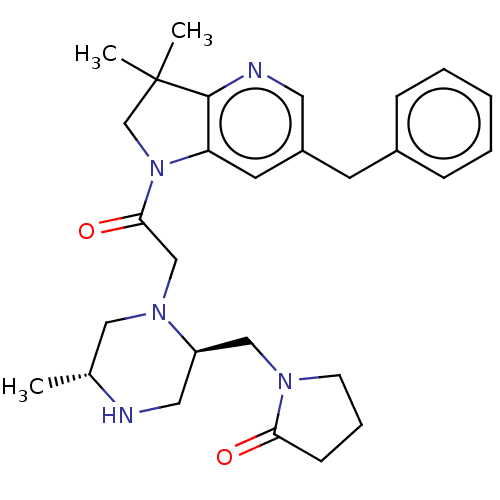 (US9018214, 180) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 44 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Astex Therapeutics Limited US Patent | Assay Description Modified SMAC peptides and compounds were tested for their ability to displace the fluorescent tracer from either XIAP, clAP-1 or clAP-2. BIR3 domain... | US Patent US9018214 (2015) BindingDB Entry DOI: 10.7270/Q23T9FXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM156129 (US9018214, 180) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Induction of cIAP1 degradation in human MDA-MB-231 cells after 2 hrs | J Med Chem 60: 4611-4625 (2017) Article DOI: 10.1021/acs.jmedchem.6b01877 BindingDB Entry DOI: 10.7270/Q2KK9DX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| X-linked inhibitor of apoptosis protein (XIAP) (Homo sapiens (Human)) | BDBM156129 (US9018214, 180) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of SMAC-derived peptide abuRPFK (5 and 6FAM)-amide interaction with XIAP BIR3 domain (unknown origin) by fluorescence polarization assay | J Med Chem 60: 4611-4625 (2017) Article DOI: 10.1021/acs.jmedchem.6b01877 BindingDB Entry DOI: 10.7270/Q2KK9DX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| X-linked inhibitor of apoptosis protein (XIAP) (Homo sapiens (Human)) | BDBM156129 (US9018214, 180) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length FLAG-tagged XIAP (unknown origin) interaction with full length untagged caspase-9 expressed in HEK293 cells after 2 hrs by ... | J Med Chem 60: 4611-4625 (2017) Article DOI: 10.1021/acs.jmedchem.6b01877 BindingDB Entry DOI: 10.7270/Q2KK9DX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||