

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM16155 1-({4-chloro-1-[(diaminomethylidene)amino]isoquinoline-7-}sulfonamido)cyclohexane-1-carboxylic acid::substituted glycine deriv. 29
SMILES: [#7]\[#6](-[#7])=[#7]/c1ncc(Cl)c2ccc(cc12)S(=O)(=O)[#7]C1([#6]-[#6]-[#6]-[#6]-[#6]1)[#6](-[#8])=O
InChI Key: InChIKey=WGQAIRAOZLFUSH-UHFFFAOYSA-N
Data: 3 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16155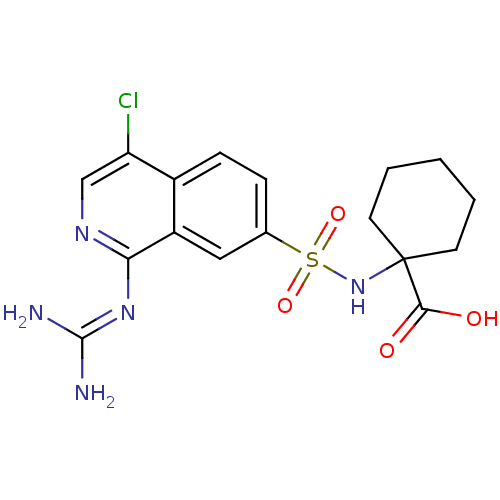 (1-({4-chloro-1-[(diaminomethylidene)amino]isoquino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM16155 (1-({4-chloro-1-[(diaminomethylidene)amino]isoquino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM16155 (1-({4-chloro-1-[(diaminomethylidene)amino]isoquino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Ki values for compounds were calculated by incubation of each enzyme with its substrate and various compound concentrations. Absorbance was read at 4... | J Med Chem 50: 2341-51 (2007) Article DOI: 10.1021/jm061066t BindingDB Entry DOI: 10.7270/Q27S7M18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||