

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM16233 4-[3-(3-nitrophenyl)-1,2,4-oxadiazol-5-yl]butanoic acid::BTB02809::Ligand 1
SMILES: OC(=O)CCCc1nc([n-][o+]1)-c1cccc(c1)[N+]([O-])=O
InChI Key: InChIKey=LQQYZJRCWBRIMW-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldose reductase (AR) (Homo sapiens (Human)) | BDBM16233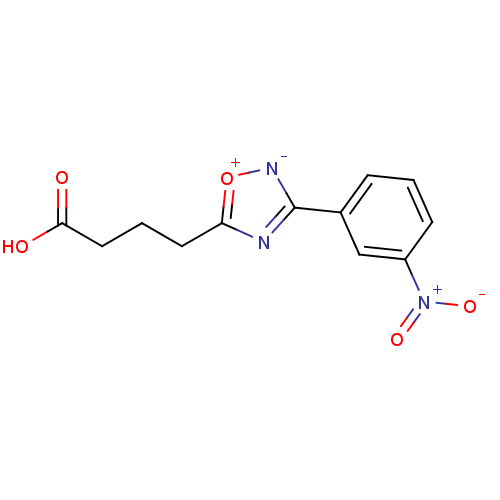 (4-[3-(3-nitrophenyl)-1,2,4-oxadiazol-5-yl]butanoic...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | 6.2 | 25 |
University of Marburg | Assay Description The in vitro inhibitory activity of the candidate molecules was determined by recording the decrease of the NADPH absorbance upon enzymatic reduction... | J Mol Biol 368: 618-38 (2007) Article DOI: 10.1016/j.jmb.2006.12.004 BindingDB Entry DOI: 10.7270/Q2GB2291 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cell (A) | Syringe (B) | Cell Links | Syringe Links | Cell + Syr Links | ΔG° kcal/mole | -TΔS° kcal/mole | ΔH° kcal/mole | log K | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|
| Aldose reductase (AR) (Homo sapiens (Human)) | BDBM16233 (4-[3-(3-nitrophenyl)-1,2,4-oxadiazol-5-yl]butanoic...) | GoogleScholar PDB | CHEBI MMDB PC cid PC sid PDB | -8.46 | -2.34 | -6.11 | 6.22 | 8 | 24.9 | |
University of Marburg | J Mol Biol 368: 618-38 (2007) | |||||||||