

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM165960 US10604504, Example 206::US9688629, 204::US9688629, 205::US9688629, 206::US9802915, Example 206::US9920031, Example 206
SMILES: Cc1[nH]c2c(cc(F)c(-c3cccc4CCN(Cc34)C(=O)C=C)c2c1C)C(N)=O
InChI Key: InChIKey=BGLCRSDXKMNCHF-UHFFFAOYSA-N
Data: 12 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165960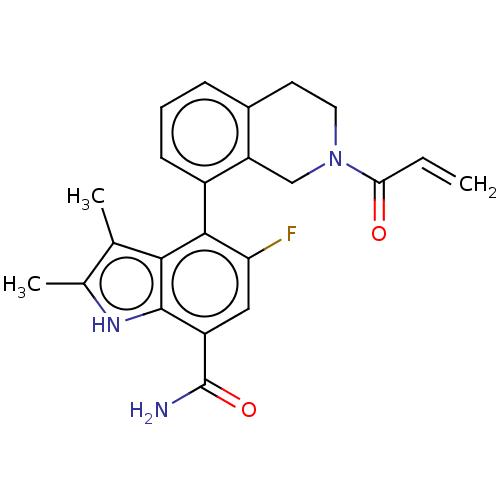 (US10604504, Example 206 | US9688629, 204 | US96886...) | PDB MMDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description To V-bottom 384-well plates were added test compounds, human recombinant Btk (1 nM, Invitrogen Corporation), fluoresceinated peptide (1.5 μM), A... | US Patent US9688629 (2017) BindingDB Entry DOI: 10.7270/Q20863G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165960 (US10604504, Example 206 | US9688629, 204 | US96886...) | PDB MMDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description To V-bottom 384-well plates were added test compounds, human recombinant Btk (1 nM, Invitrogen Corporation), fluoresceinated peptide (1.5 μM), A... | US Patent US9688629 (2017) BindingDB Entry DOI: 10.7270/Q20863G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165960 (US10604504, Example 206 | US9688629, 204 | US96886...) | PDB MMDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description To V-bottom 384-well plates were added test compounds, human recombinant Btk (1 nM, Invitrogen Corporation), fluoresceinated peptide (1.5 μM), A... | US Patent US9688629 (2017) BindingDB Entry DOI: 10.7270/Q20863G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165960 (US10604504, Example 206 | US9688629, 204 | US96886...) | PDB MMDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To V-bottom 384-well plates were added test compounds, human recombinant Btk (1 nM, Invitrogen Corporation), fluoresceinated peptide (1.5 μM), A... | US Patent US9802915 (2017) BindingDB Entry DOI: 10.7270/Q2JW8H17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165960 (US10604504, Example 206 | US9688629, 204 | US96886...) | PDB MMDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To V-bottom 384-well plates were added test compounds, human recombinant Btk (1 nM, Invitrogen Corporation), fluoresceinated peptide (1.5 μM), A... | US Patent US9802915 (2017) BindingDB Entry DOI: 10.7270/Q2JW8H17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165960 (US10604504, Example 206 | US9688629, 204 | US96886...) | PDB MMDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To V-bottom 384-well plates were added test compounds, human recombinant Btk (1 nM, Invitrogen Corporation), fluoresceinated peptide (1.5 μM), A... | US Patent US10604504 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165960 (US10604504, Example 206 | US9688629, 204 | US96886...) | PDB MMDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description To V-bottom 384-well plates were added test compounds, human recombinant Btk (1 nM, Invitrogen Corporation), fluoresceinated peptide (1.5 μM), A... | J Med Chem 50: 4928-38 (2007) BindingDB Entry DOI: 10.7270/Q2474D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165960 (US10604504, Example 206 | US9688629, 204 | US96886...) | PDB MMDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description To V-bottom 384-well plates were added test compounds, human recombinant Btk (1 nM, Invitrogen Corporation), fluoresceinated peptide (1.5 μM), A... | J Med Chem 50: 4928-38 (2007) BindingDB Entry DOI: 10.7270/Q2474D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165960 (US10604504, Example 206 | US9688629, 204 | US96886...) | PDB MMDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description To V-bottom 384-well plates were added test compounds, human recombinant Btk (1 nM, Invitrogen Corporation), fluoresceinated peptide (1.5 μM), A... | J Med Chem 50: 4928-38 (2007) BindingDB Entry DOI: 10.7270/Q2474D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165960 (US10604504, Example 206 | US9688629, 204 | US96886...) | PDB MMDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To V-bottom 384-well plates were added test compounds, human recombinant Btk (1 nM, Invitrogen Corporation), fluoresceinated peptide (1.5 μM), A... | US Patent US10604504 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165960 (US10604504, Example 206 | US9688629, 204 | US96886...) | PDB MMDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To V-bottom 384-well plates were added test compounds, human recombinant Btk (1 nM, Invitrogen Corporation), fluoresceinated peptide (1.5 μM), A... | US Patent US10604504 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM165960 (US10604504, Example 206 | US9688629, 204 | US96886...) | PDB MMDB NCI pathway Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description To V-bottom 384-well plates were added test compounds, human recombinant Btk (1 nM, Invitrogen Corporation), fluoresceinated peptide (1.5 μM), A... | US Patent US9802915 (2017) BindingDB Entry DOI: 10.7270/Q2JW8H17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||