

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM172164 US10155002, Compound 172::US9090562, 172
SMILES: CCN([C@H]1CC[C@@H](CC1)N(C)C)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)-c1ccc(nc1)N1CCNCC1
InChI Key:
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172164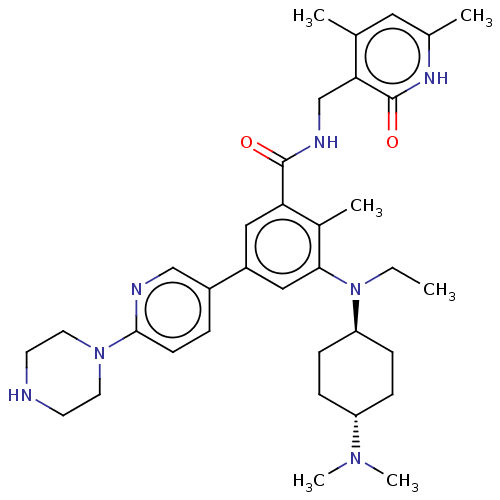 (US10155002, Compound 172 | US11052093, Compound 17...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.26 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| EZH2(Y641F) (Homo sapiens (Human)) | BDBM172164 (US10155002, Compound 172 | US11052093, Compound 17...) | PDB MMDB KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.21 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9090562 (2015) BindingDB Entry DOI: 10.7270/Q2057DPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| EZH2(Y641F) (Homo sapiens (Human)) | BDBM172164 (US10155002, Compound 172 | US11052093, Compound 17...) | PDB MMDB KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 4.21 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| EZH2(Y641F) (Homo sapiens (Human)) | BDBM172164 (US10155002, Compound 172 | US11052093, Compound 17...) | PDB MMDB KEGG B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.21 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172164 (US10155002, Compound 172 | US11052093, Compound 17...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 9.26 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172164 (US10155002, Compound 172 | US11052093, Compound 17...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.26 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US10155002 (2018) BindingDB Entry DOI: 10.7270/Q2KS6TM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||