

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM17462 1,2,3-triazole analogue, 18::5-(3-methylphenyl)-1H-1,2,3-triazole::CHEMBL194853
SMILES: Cc1cccc(c1)-c1c[nH]nn1
InChI Key: InChIKey=XQHCBHNLRWLGQS-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17462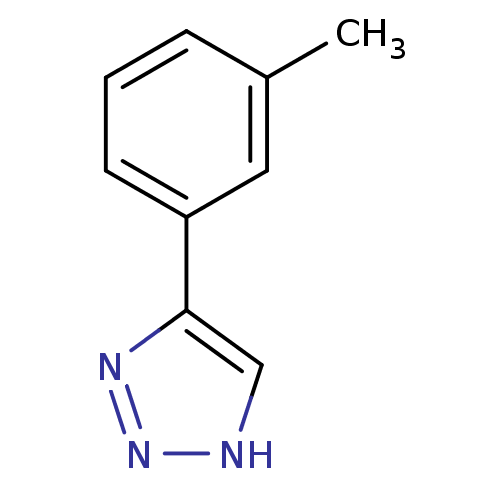 (1,2,3-triazole analogue, 18 | 5-(3-methylphenyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 18 | -10.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM17462 (1,2,3-triazole analogue, 18 | 5-(3-methylphenyl)-1...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase (Homo sapiens (Human)) | BDBM17462 (1,2,3-triazole analogue, 18 | 5-(3-methylphenyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His-tagged IDO1 (Ala2 to Gly403) overexpressed in Escherichia coli BL21 at pH 6.5 after 60 mins by HPLC an... | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Mus musculus) | BDBM17462 (1,2,3-triazole analogue, 18 | 5-(3-methylphenyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse TDO in P815 clone 12 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM17462 (1,2,3-triazole analogue, 18 | 5-(3-methylphenyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human TDO transfected in mouse P815B clone 19 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17462 (1,2,3-triazole analogue, 18 | 5-(3-methylphenyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Therapeutics Ltd. Curated by ChEMBL | Assay Description Inhibition of MetAp2 | J Med Chem 51: 3661-80 (2008) Article DOI: 10.1021/jm8000373 BindingDB Entry DOI: 10.7270/Q2N58M4H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase (Homo sapiens (Human)) | BDBM17462 (1,2,3-triazole analogue, 18 | 5-(3-methylphenyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human IDO1 transfected in mouse P815B clone-6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||