

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM17966 (6R)-1-(4-{3-[(2-methoxyphenyl)methoxy]propoxy}phenyl)-6-[(naphthalen-2-yloxy)methyl]piperazin-2-one::CHEMBL371426::Ketopiperazine-based compound, 2
SMILES: COc1ccccc1COCCCOc1ccc(cc1)N1[C@@H](COc2ccc3ccccc3c2)CNCC1=O
InChI Key: InChIKey=YNZFVUGNLOWSTD-MUUNZHRXSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM17966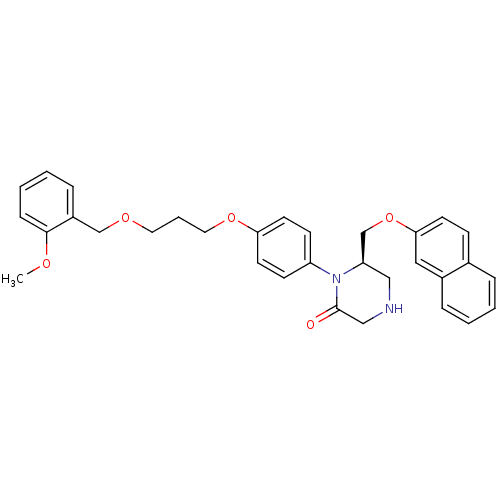 ((6R)-1-(4-{3-[(2-methoxyphenyl)methoxy]propoxy}phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The renin assay utilized a tandem Green Flourescent Protein (tGFP) substrate that was hydrolyzed by human rennin. The tGFP substrate contained a nine... | Bioorg Med Chem 13: 2657-64 (2005) Article DOI: 10.1016/j.bmc.2005.01.048 BindingDB Entry DOI: 10.7270/Q2KP80D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17966 ((6R)-1-(4-{3-[(2-methoxyphenyl)methoxy]propoxy}phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corp Curated by ChEMBL | Assay Description Inhibition of human recombinant renin in buffer | J Med Chem 53: 7490-520 (2010) Article DOI: 10.1021/jm901885s BindingDB Entry DOI: 10.7270/Q2S75GKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17966 ((6R)-1-(4-{3-[(2-methoxyphenyl)methoxy]propoxy}phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Concentration required to inhibit renin activity by 50% | Bioorg Med Chem Lett 15: 2371-4 (2005) Article DOI: 10.1016/j.bmcl.2005.02.085 BindingDB Entry DOI: 10.7270/Q2PV6JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||