

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM18197 1-(2,2,2-trifluoroethyl)-9-(trifluoromethyl)-1H,2H,3H,6H,7H-quinolino[7,6-b][1,4]oxazin-7-one::7H-[1,4]Oxazino[3,2-g]quinolin-7-one based compound, 10f
SMILES: FC(F)(F)CN1CCOc2cc3[nH]c(=O)cc(c3cc12)C(F)(F)F
InChI Key: InChIKey=DKTPAKMQCCCINA-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Androgen Receptor (Homo sapiens (Human)) | BDBM18197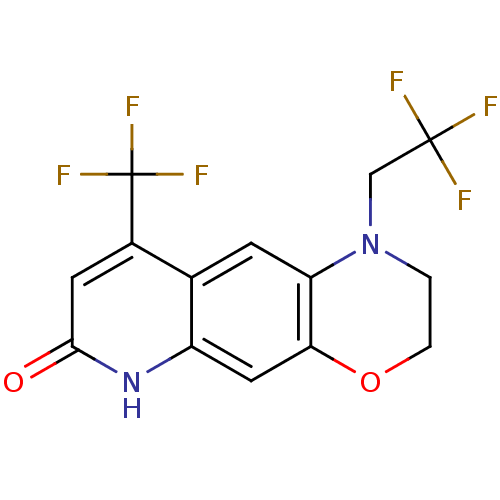 (1-(2,2,2-trifluoroethyl)-9-(trifluoromethyl)-1H,2H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | -11.9 | n/a | n/a | 3.5 | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | J Med Chem 50: 2486-96 (2007) Article DOI: 10.1021/jm061329j BindingDB Entry DOI: 10.7270/Q20R9MNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18197 (1-(2,2,2-trifluoroethyl)-9-(trifluoromethyl)-1H,2H...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5.30E+3 | -6.69 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I... | J Med Chem 50: 2486-96 (2007) Article DOI: 10.1021/jm061329j BindingDB Entry DOI: 10.7270/Q20R9MNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18197 (1-(2,2,2-trifluoroethyl)-9-(trifluoromethyl)-1H,2H...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 9.20E+3 | -6.38 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I... | J Med Chem 50: 2486-96 (2007) Article DOI: 10.1021/jm061329j BindingDB Entry DOI: 10.7270/Q20R9MNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||