

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM18213 2-methyl-1-(2,2,2-trifluoroethyl)-9-(trifluoromethyl)-1H,2H,3H,6H,7H-quinolino[7,6-b][1,4]oxazin-7-one::7H-[1,4]Oxazino[3,2-g]quinolin-7-one based compound, 16e
SMILES: CC1COc2cc3[nH]c(=O)cc(c3cc2N1CC(F)(F)F)C(F)(F)F
InChI Key: InChIKey=SPBFNRFRJLBCIQ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Androgen Receptor (Homo sapiens (Human)) | BDBM18213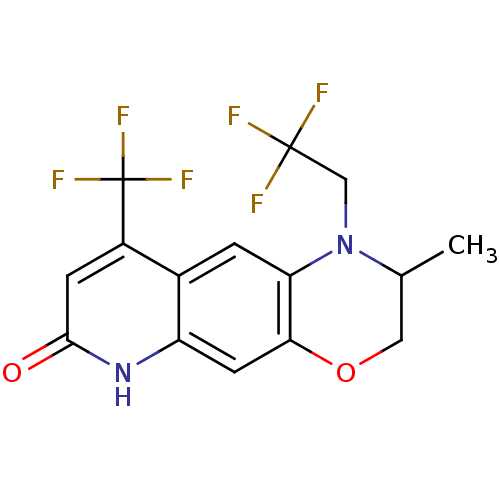 (2-methyl-1-(2,2,2-trifluoroethyl)-9-(trifluorometh...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 7.80 | n/a | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | J Med Chem 50: 2486-96 (2007) Article DOI: 10.1021/jm061329j BindingDB Entry DOI: 10.7270/Q20R9MNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18213 (2-methyl-1-(2,2,2-trifluoroethyl)-9-(trifluorometh...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I... | J Med Chem 50: 2486-96 (2007) Article DOI: 10.1021/jm061329j BindingDB Entry DOI: 10.7270/Q20R9MNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18213 (2-methyl-1-(2,2,2-trifluoroethyl)-9-(trifluorometh...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I... | J Med Chem 50: 2486-96 (2007) Article DOI: 10.1021/jm061329j BindingDB Entry DOI: 10.7270/Q20R9MNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||