

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM185674 4-[4-[(5-tert-butyl-2-quinolin-6-ylpyrazol-3-yl)carbamoylamino]-3-fluorophenoxy]-N-methylpyridine-2-carboxamide::Rebastinib
SMILES: CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3cc(nn3-c3ccc4ncccc4c3)C(C)(C)C)c(F)c2)ccn1
InChI Key: InChIKey=WVXNSAVVKYZVOE-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase ABL1 [229-512] (Homo sapiens (Human)) | BDBM185674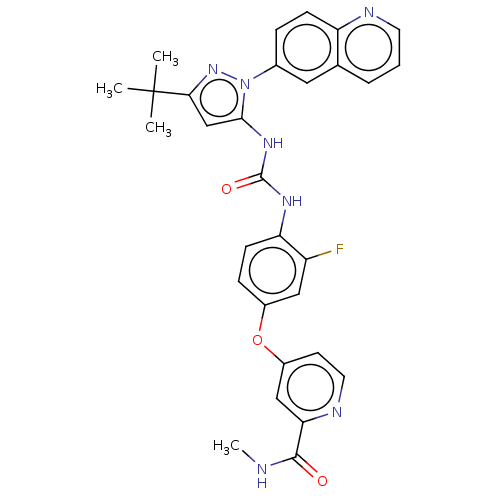 (4-[4-[(5-tert-butyl-2-quinolin-6-ylpyrazol-3-yl)ca...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a |
Stony Brook University | Assay Description Inhibitor selectivity profiles were obtained through Luceome Biotechnologies (Tuscon, AZ). Each inhibitor was screened at 0.5 μM against a panel... | ACS Chem Biol 11: 1296-304 (2016) Article DOI: 10.1021/acschembio.5b01018 BindingDB Entry DOI: 10.7270/Q2MW2FXD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM185674 (4-[4-[(5-tert-butyl-2-quinolin-6-ylpyrazol-3-yl)ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research Curated by ChEMBL | Assay Description Inhibition of Yes1 (unknown origin) by [gamma-33P]-ATP radiolabeled enzyme activity assay | Bioorg Med Chem Lett 23: 4398-403 (2013) Article DOI: 10.1016/j.bmcl.2013.05.072 BindingDB Entry DOI: 10.7270/Q2BR8W3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM185674 (4-[4-[(5-tert-butyl-2-quinolin-6-ylpyrazol-3-yl)ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research Curated by ChEMBL | Assay Description Inhibition of Yes1 (unknown origin) assessed as kinase-dependent enzymatic production of ADP from ATP using coupled luminescence-based reaction by AD... | Bioorg Med Chem Lett 23: 4398-403 (2013) Article DOI: 10.1016/j.bmcl.2013.05.072 BindingDB Entry DOI: 10.7270/Q2BR8W3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src [251-533] (Gallus gallus (Chicken)) | BDBM185674 (4-[4-[(5-tert-butyl-2-quinolin-6-ylpyrazol-3-yl)ca...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a |
Stony Brook University | Assay Description Inhibitor selectivity profiles were obtained through Luceome Biotechnologies (Tuscon, AZ). Each inhibitor was screened at 0.5 μM against phospho... | ACS Chem Biol 11: 1296-304 (2016) Article DOI: 10.1021/acschembio.5b01018 BindingDB Entry DOI: 10.7270/Q2MW2FXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||