

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM19762 (2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-2-[2-(4-chlorophenoxy)-2-methylpropanamido]-4-methylpentanamide::dipeptidyl nitrile, 16c
SMILES: CC(C)C[C@H](NC(=O)C(C)(C)Oc1ccc(Cl)cc1)C(=O)N[C@@H](COCc1ccccc1)C#N
InChI Key: InChIKey=AMJZLJJCJXONIE-GGAORHGYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Homo sapiens (Human)) | BDBM19762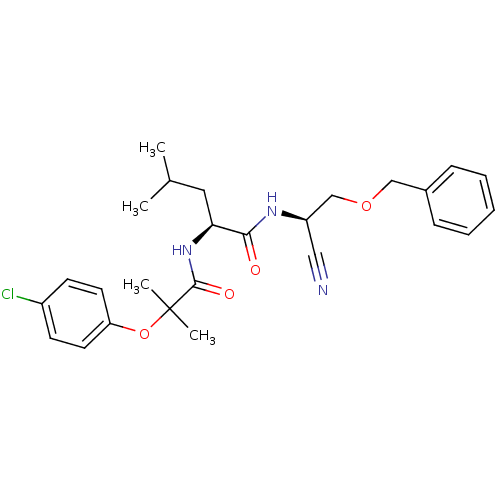 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-2-[2-(4-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19762 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-2-[2-(4-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19762 ((2S)-N-[(1R)-2-(benzyloxy)-1-cyanoethyl]-2-[2-(4-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <30 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Novartis Pharmaceuticals | Assay Description The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... | Bioorg Med Chem Lett 16: 2549-54 (2006) Article DOI: 10.1016/j.bmcl.2006.01.104 BindingDB Entry DOI: 10.7270/Q2XG9PFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||