 Found 4 hits for monomerid = 203661
Found 4 hits for monomerid = 203661 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM203661
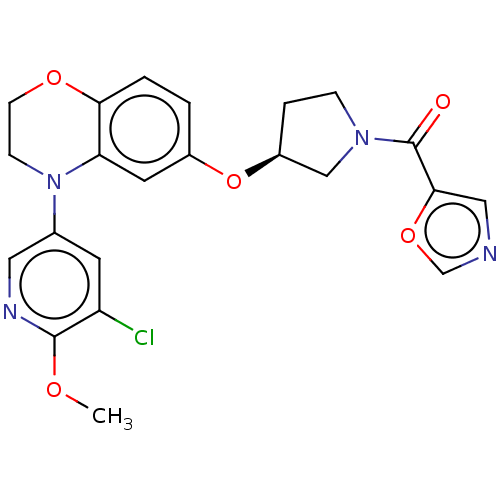
(US9539260, B108 | US9763952, Example B108)Show SMILES COc1ncc(cc1Cl)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)c3cnco3)cc12 |r| Show InChI InChI=1S/C22H21ClN4O5/c1-29-21-17(23)8-14(10-25-21)27-6-7-30-19-3-2-15(9-18(19)27)32-16-4-5-26(12-16)22(28)20-11-24-13-31-20/h2-3,8-11,13,16H,4-7,12H2,1H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1... |
US Patent US9539260 (2017)
BindingDB Entry DOI: 10.7270/Q2W37TH1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM203661

(US9539260, B108 | US9763952, Example B108)Show SMILES COc1ncc(cc1Cl)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)c3cnco3)cc12 |r| Show InChI InChI=1S/C22H21ClN4O5/c1-29-21-17(23)8-14(10-25-21)27-6-7-30-19-3-2-15(9-18(19)27)32-16-4-5-26(12-16)22(28)20-11-24-13-31-20/h2-3,8-11,13,16H,4-7,12H2,1H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM203661

(US9539260, B108 | US9763952, Example B108)Show SMILES COc1ncc(cc1Cl)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)c3cnco3)cc12 |r| Show InChI InChI=1S/C22H21ClN4O5/c1-29-21-17(23)8-14(10-25-21)27-6-7-30-19-3-2-15(9-18(19)27)32-16-4-5-26(12-16)22(28)20-11-24-13-31-20/h2-3,8-11,13,16H,4-7,12H2,1H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| Assay Description
The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The final concentrations of ATP and p... |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM203661

(US9539260, B108 | US9763952, Example B108)Show SMILES COc1ncc(cc1Cl)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)c3cnco3)cc12 |r| Show InChI InChI=1S/C22H21ClN4O5/c1-29-21-17(23)8-14(10-25-21)27-6-7-30-19-3-2-15(9-18(19)27)32-16-4-5-26(12-16)22(28)20-11-24-13-31-20/h2-3,8-11,13,16H,4-7,12H2,1H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1... |
US Patent US9539260 (2017)
BindingDB Entry DOI: 10.7270/Q2W37TH1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data