 Found 13 hits for monomerid = 205420
Found 13 hits for monomerid = 205420 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
N-acetyl-beta-D-glucosaminidase (O-GlcNAcase)
(Homo sapiens (Human)) | BDBM205420
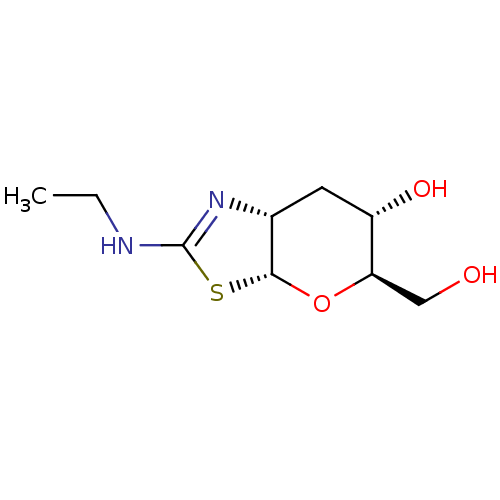
(US9243020, 2 | US9815861, Example 2)Show SMILES CCNC1=N[C@@H]2C[C@H](O)[C@@H](CO)O[C@@H]2S1 |r,t:3| Show InChI InChI=1S/C9H16N2O3S/c1-2-10-9-11-5-3-6(13)7(4-12)14-8(5)15-9/h5-8,12-13H,2-4H2,1H3,(H,10,11)/t5-,6+,7-,8-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 44 | -10.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Alectos Therapeutics Inc.; Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... |
US Patent US9243020 (2016)
BindingDB Entry DOI: 10.7270/Q2542MD4 |
More data for this
Ligand-Target Pair | |
N-acetyl-beta-D-glucosaminidase (O-GlcNAcase)
(Homo sapiens (Human)) | BDBM205420

(US9243020, 2 | US9815861, Example 2)Show SMILES CCNC1=N[C@@H]2C[C@H](O)[C@@H](CO)O[C@@H]2S1 |r,t:3| Show InChI InChI=1S/C9H16N2O3S/c1-2-10-9-11-5-3-6(13)7(4-12)14-8(5)15-9/h5-8,12-13H,2-4H2,1H3,(H,10,11)/t5-,6+,7-,8-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human OGA |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090 |
More data for this
Ligand-Target Pair | |
N-acetyl-beta-D-glucosaminidase (O-GlcNAcase)
(Homo sapiens (Human)) | BDBM205420

(US9243020, 2 | US9815861, Example 2)Show SMILES CCNC1=N[C@@H]2C[C@H](O)[C@@H](CO)O[C@@H]2S1 |r,t:3| Show InChI InChI=1S/C9H16N2O3S/c1-2-10-9-11-5-3-6(13)7(4-12)14-8(5)15-9/h5-8,12-13H,2-4H2,1H3,(H,10,11)/t5-,6+,7-,8-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alectos Therapeutics, Inc.; Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... |
US Patent US9815861 (2017)
BindingDB Entry DOI: 10.7270/Q2R78HJ8 |
More data for this
Ligand-Target Pair | |
Beta-hexosaminidase subunit beta (Hex B)
(Homo sapiens (Human)) | BDBM205420

(US9243020, 2 | US9815861, Example 2)Show SMILES CCNC1=N[C@@H]2C[C@H](O)[C@@H](CO)O[C@@H]2S1 |r,t:3| Show InChI InChI=1S/C9H16N2O3S/c1-2-10-9-11-5-3-6(13)7(4-12)14-8(5)15-9/h5-8,12-13H,2-4H2,1H3,(H,10,11)/t5-,6+,7-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090 |
More data for this
Ligand-Target Pair | |
Pregnane X receptor
(Homo sapiens (Human)) | BDBM205420

(US9243020, 2 | US9815861, Example 2)Show SMILES CCNC1=N[C@@H]2C[C@H](O)[C@@H](CO)O[C@@H]2S1 |r,t:3| Show InChI InChI=1S/C9H16N2O3S/c1-2-10-9-11-5-3-6(13)7(4-12)14-8(5)15-9/h5-8,12-13H,2-4H2,1H3,(H,10,11)/t5-,6+,7-,8-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Activation of PXR (unknown origin) assessed as CYP3A4 induction |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090 |
More data for this
Ligand-Target Pair | |
L-type calcium channel alpha-1c/beta-2/alpha2delta-1
(Homo sapiens (Human)) | BDBM205420

(US9243020, 2 | US9815861, Example 2)Show SMILES CCNC1=N[C@@H]2C[C@H](O)[C@@H](CO)O[C@@H]2S1 |r,t:3| Show InChI InChI=1S/C9H16N2O3S/c1-2-10-9-11-5-3-6(13)7(4-12)14-8(5)15-9/h5-8,12-13H,2-4H2,1H3,(H,10,11)/t5-,6+,7-,8-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of Cav1.2 (unknown origin) |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM205420

(US9243020, 2 | US9815861, Example 2)Show SMILES CCNC1=N[C@@H]2C[C@H](O)[C@@H](CO)O[C@@H]2S1 |r,t:3| Show InChI InChI=1S/C9H16N2O3S/c1-2-10-9-11-5-3-6(13)7(4-12)14-8(5)15-9/h5-8,12-13H,2-4H2,1H3,(H,10,11)/t5-,6+,7-,8-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.5 (unknown origin) |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM205420

(US9243020, 2 | US9815861, Example 2)Show SMILES CCNC1=N[C@@H]2C[C@H](O)[C@@H](CO)O[C@@H]2S1 |r,t:3| Show InChI InChI=1S/C9H16N2O3S/c1-2-10-9-11-5-3-6(13)7(4-12)14-8(5)15-9/h5-8,12-13H,2-4H2,1H3,(H,10,11)/t5-,6+,7-,8-/m1/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090 |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Rattus norvegicus) | BDBM205420

(US9243020, 2 | US9815861, Example 2)Show SMILES CCNC1=N[C@@H]2C[C@H](O)[C@@H](CO)O[C@@H]2S1 |r,t:3| Show InChI InChI=1S/C9H16N2O3S/c1-2-10-9-11-5-3-6(13)7(4-12)14-8(5)15-9/h5-8,12-13H,2-4H2,1H3,(H,10,11)/t5-,6+,7-,8-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 364 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of OGA in rat PC12 cells assessed as OGlcNAcylated protein level incubated for 24 hrs by ELISA |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM205420

(US9243020, 2 | US9815861, Example 2)Show SMILES CCNC1=N[C@@H]2C[C@H](O)[C@@H](CO)O[C@@H]2S1 |r,t:3| Show InChI InChI=1S/C9H16N2O3S/c1-2-10-9-11-5-3-6(13)7(4-12)14-8(5)15-9/h5-8,12-13H,2-4H2,1H3,(H,10,11)/t5-,6+,7-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090 |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase component GLS2
(Saccharomyces cerevisiae) | BDBM205420

(US9243020, 2 | US9815861, Example 2)Show SMILES CCNC1=N[C@@H]2C[C@H](O)[C@@H](CO)O[C@@H]2S1 |r,t:3| Show InChI InChI=1S/C9H16N2O3S/c1-2-10-9-11-5-3-6(13)7(4-12)14-8(5)15-9/h5-8,12-13H,2-4H2,1H3,(H,10,11)/t5-,6+,7-,8-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of MK-0499 from human ERG |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090 |
More data for this
Ligand-Target Pair | |
N-acetyl-beta-D-glucosaminidase (O-GlcNAcase)
(Homo sapiens (Human)) | BDBM205420

(US9243020, 2 | US9815861, Example 2)Show SMILES CCNC1=N[C@@H]2C[C@H](O)[C@@H](CO)O[C@@H]2S1 |r,t:3| Show InChI InChI=1S/C9H16N2O3S/c1-2-10-9-11-5-3-6(13)7(4-12)14-8(5)15-9/h5-8,12-13H,2-4H2,1H3,(H,10,11)/t5-,6+,7-,8-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 365 | n/a | n/a | n/a | 4 |
Alectos Therapeutics Inc.; Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The ELISA portion of the assay was performed in a black Maxisorp 96-well plate that was coated overnight at 4° C. with 100 uL/well of the cell ly... |
US Patent US9243020 (2016)
BindingDB Entry DOI: 10.7270/Q2542MD4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM205420

(US9243020, 2 | US9815861, Example 2)Show SMILES CCNC1=N[C@@H]2C[C@H](O)[C@@H](CO)O[C@@H]2S1 |r,t:3| Show InChI InChI=1S/C9H16N2O3S/c1-2-10-9-11-5-3-6(13)7(4-12)14-8(5)15-9/h5-8,12-13H,2-4H2,1H3,(H,10,11)/t5-,6+,7-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data